Sphingosine kinase 1 activation enhances epidermal innate immunity through sphingosine-1-phosphate stimulation of cathelicidin production
- PMID: 26113114
- PMCID: PMC4624019
- DOI: 10.1016/j.jdermsci.2015.06.007
Sphingosine kinase 1 activation enhances epidermal innate immunity through sphingosine-1-phosphate stimulation of cathelicidin production
Abstract
Background: The ceramide metabolite, sphingosine-1-phosphate (S1P), regulates multiple cellular functions in keratinocytes (KC). We recently discovered that production of a key innate immune element, cathelicidin antimicrobial peptide (CAMP), is stimulated via a NF-κB-dependent mechanism that is activated by S1P when S1P is generated by sphingosine kinase (SPHK) 1.
Objective: We investigated whether pharmacological modulation of SPHK1 activity, using a novel synthetic SPHK1 activator, (S)-methyl 2-(hexanamide)-3-(4-hydroxyphenyl) propanoate (MHP), stimulates CAMP expression.
Methods: MHP-mediated changes in both S1P and CAMP downstream mediators were analyzed in normal cultured human KC by qRT-PCR, Western immunoblot, ELISA, confocal microscopy for immunohistochemistry, HPLC and ESI-LC/MS/MS, and microbial pathogen invasion/colonization in a human epidermal organotypic model.
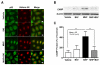
Results: Treatment with MHP directly activated SPHK1 and increased cellular S1P content in normal cultured human KC. Because MHP did not inhibit S1P lyase activity, which hydrolyses S1P, augumented S1P levels could be attributed to increased synthesis rather than blockade of S1P degradation. Next, we found that exogenous MHP significantly stimulated CAMP mRNA and protein production in KC, increases that were significantly suppressed by siRNA directed against SPHK1, but not by a scrambled control siRNA. NF-κB activation, assessed by nuclear translocation of NF-κB, occurred in cells following incubation with MHP. Conversely, pretreatment with a specific inhibitor of SPHK1 decreased MHP-induced nuclear translocation of NF-κB, and significantly attenuated the MHP-mediated increase in CAMP production. Finally, topical MHP significantly suppressed invasion of the virulent Staphylococcus aureus into murine skin explants.
Conclusion: MHP activation of SPHK1, a target enzyme of CAMP production, can stimulate innate immunity.
Keywords: Antimicrobial defense; Cathelicidin antimicrobial peptide; Small molecule; Sphingosine kinase 1.
Copyright © 2015 Elsevier Ireland Ltd. All rights reserved.
Figures



Similar articles
-
Resveratrol stimulates sphingosine-1-phosphate signaling of cathelicidin production.J Invest Dermatol. 2013 Aug;133(8):1942-9. doi: 10.1038/jid.2013.133. Epub 2013 Mar 14. J Invest Dermatol. 2013. PMID: 23856934 Free PMC article.
-
A novel role of a lipid species, sphingosine-1-phosphate, in epithelial innate immunity.Mol Cell Biol. 2013 Feb;33(4):752-62. doi: 10.1128/MCB.01103-12. Epub 2012 Dec 10. Mol Cell Biol. 2013. PMID: 23230267 Free PMC article.
-
ER stress stimulates production of the key antimicrobial peptide, cathelicidin, by forming a previously unidentified intracellular S1P signaling complex.Proc Natl Acad Sci U S A. 2016 Mar 8;113(10):E1334-42. doi: 10.1073/pnas.1504555113. Epub 2016 Feb 22. Proc Natl Acad Sci U S A. 2016. PMID: 26903652 Free PMC article.
-
Sphingosine kinase 1 in viral infections.Rev Med Virol. 2013 Mar;23(2):73-84. doi: 10.1002/rmv.1718. Epub 2012 May 28. Rev Med Virol. 2013. PMID: 22639116 Review.
-
The sphingosine kinase-1/sphingosine-1-phosphate axis in cancer: Potential target for anticancer therapy.Pharmacol Ther. 2019 Mar;195:85-99. doi: 10.1016/j.pharmthera.2018.10.011. Epub 2018 Oct 19. Pharmacol Ther. 2019. PMID: 30347210 Review.
Cited by
-
Sphingosine-1-phosphate in inflammatory bowel disease and colitis-associated colon cancer: the fat's in the fire.Transl Cancer Res. 2015 Oct 1;4(5):469-483. doi: 10.3978/j.issn.2218-676X.2015.10.06. Transl Cancer Res. 2015. PMID: 27011900 Free PMC article.
-
Efficacy of a Multi-lamellar Emulsion Containing a Synthetic Sphingosine Kinase 1 Activator and Pseudoceramide in Patients with Atopic Dermatitis: A Randomized Controlled Trial.Dermatol Ther (Heidelb). 2024 Sep;14(9):2591-2605. doi: 10.1007/s13555-024-01254-5. Epub 2024 Aug 30. Dermatol Ther (Heidelb). 2024. PMID: 39212849 Free PMC article.
-
Upregulating Human Cathelicidin Antimicrobial Peptide LL-37 Expression May Prevent Severe COVID-19 Inflammatory Responses and Reduce Microthrombosis.Front Immunol. 2022 May 12;13:880961. doi: 10.3389/fimmu.2022.880961. eCollection 2022. Front Immunol. 2022. PMID: 35634307 Free PMC article. Review.
-
Abnormal Sphingolipid World in Inflammation Specific for Lysosomal Storage Diseases and Skin Disorders.Int J Mol Sci. 2018 Jan 15;19(1):247. doi: 10.3390/ijms19010247. Int J Mol Sci. 2018. PMID: 29342918 Free PMC article. Review.
-
Atopic dermatitis: an expanding therapeutic pipeline for a complex disease.Nat Rev Drug Discov. 2022 Jan;21(1):21-40. doi: 10.1038/s41573-021-00266-6. Epub 2021 Aug 20. Nat Rev Drug Discov. 2022. PMID: 34417579 Free PMC article. Review.
References
-
- Elias PM. Stratum corneum defensive functions: an integrated view. J Invest Dermatol. 2005;125:183–200. - PubMed
-
- Schauber J, Gallo RL. Antimicrobial peptides and the skin immune defense system. J Allergy Clin Immunol. 2009;124:R13–8. - PubMed
-
- Mendez-Samperio P. The human cathelicidin hCAP18/LL-37: a multifunctional peptide involved in mycobacterial infections. Peptides. 2010;31:1791–8. - PubMed
-
- Schroder JM. The role of keratinocytes in defense against infection. Curr Opin Infect Dis. 2010;23:106–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

