Acute and subchronic exposure to air particulate matter induces expression of angiotensin and bradykinin-related genes in the lungs and heart: Angiotensin-II type-I receptor as a molecular target of particulate matter exposure
- PMID: 26113123
- PMCID: PMC4482198
- DOI: 10.1186/s12989-015-0094-4
Acute and subchronic exposure to air particulate matter induces expression of angiotensin and bradykinin-related genes in the lungs and heart: Angiotensin-II type-I receptor as a molecular target of particulate matter exposure
Abstract
Background: Particulate matter (PM) adverse effects on health include lung and heart damage. The renin-angiotensin-aldosterone (RAAS) and kallikrein-kinin (KKS) endocrine systems are involved in the pathophysiology of cardiovascular diseases and have been found to impact lung diseases. The aim of the present study was to evaluate whether PM exposure regulates elements of RAAS and KKS.
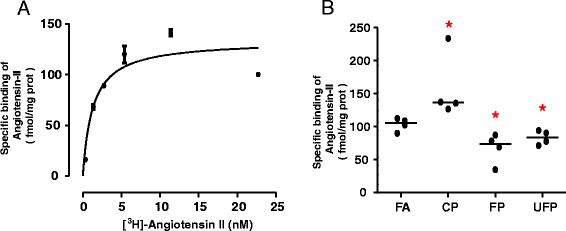
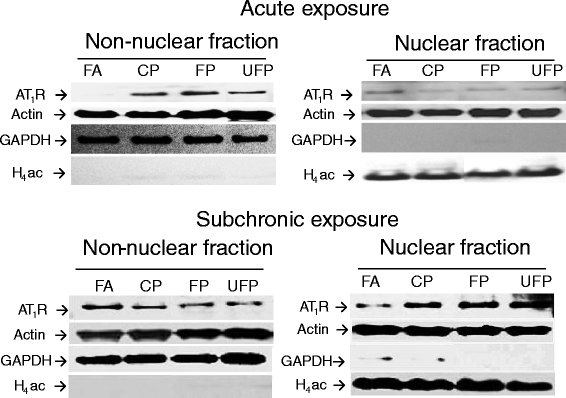
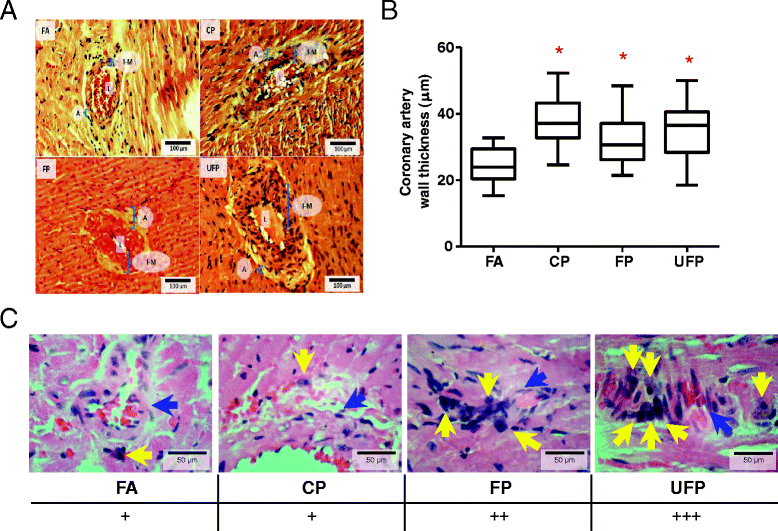
Methods: Sprague-Dawley rats were acutely (3 days) and subchronically (8 weeks) exposed to coarse (CP), fine (FP) or ultrafine (UFP) particulates using a particulate concentrator, and a control group exposed to filtered air (FA). We evaluated the mRNA of the RAAS components At1, At2r and Ace, and of the KKS components B1r, B2r and Klk-1 by RT-PCR in the lungs and heart. The ACE and AT1R protein were evaluated by Western blot, as were HO-1 and γGCSc as indicators of the antioxidant response and IL-6 levels as an inflammation marker. We performed a binding assay to determinate AT1R density in the lung, also the subcellular AT1R distribution in the lungs was evaluated. Finally, we performed a histological analysis of intramyocardial coronary arteries and the expression of markers of heart gene reprogramming (Acta1 and Col3a1).
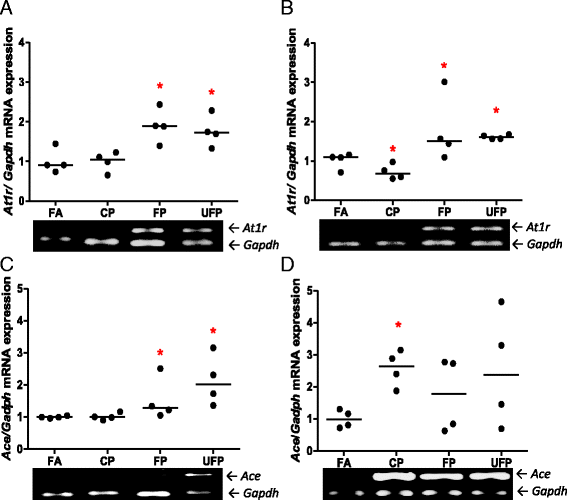
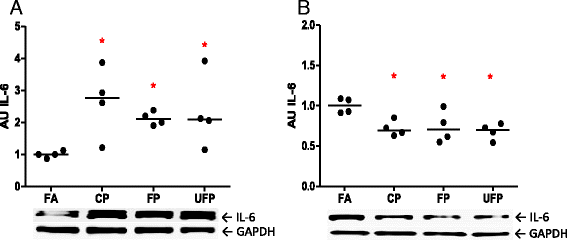
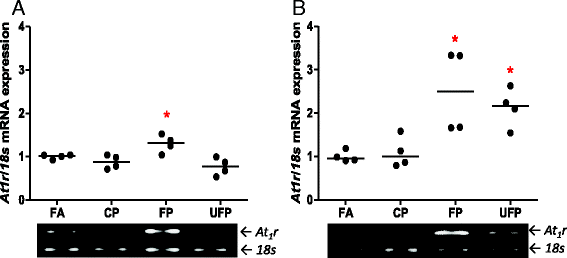
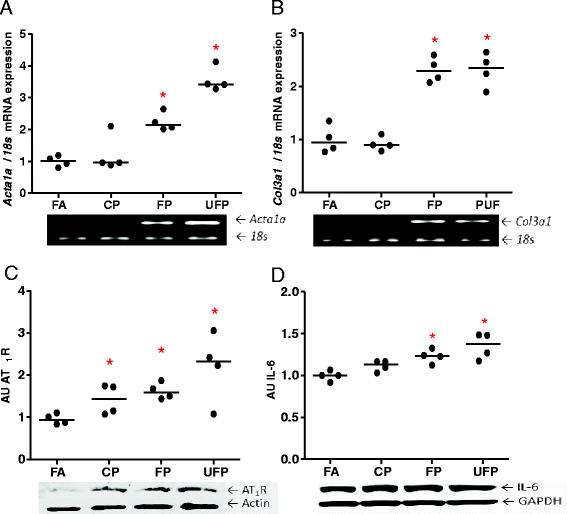
Results: The PM fractions induced the expression of RAAS and KKS elements in the lungs and heart in a time-dependent manner. CP exposure induced Ace mRNA expression and regulated its protein in the lungs. Acute and subchronic exposure to FP and UFP induced the expression of At1r in the lungs and heart. All PM fractions increased the AT1R protein in a size-dependent manner in the lungs and heart after subchronic exposure. The AT1R lung protein showed a time-dependent change in subcellular distribution. In addition, the presence of AT1R in the heart was accompanied by a decrease in HO-1, which was concomitant with the induction of Acta1 and Col3a1 and the increment of IL-6. Moreover, exposure to all PM fractions increased coronary artery wall thickness.
Conclusion: We demonstrate that exposure to PM induces the expression of RAAS and KKS elements, including AT1R, which was the main target in the lungs and the heart.
Figures


References
-
- Pope CA, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, et al. Cardiovascular mortality and long-term exposure to particulate air pollution epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

