Molecular pathogenesis of retinal and choroidal vascular diseases
- PMID: 26113211
- PMCID: PMC4651818
- DOI: 10.1016/j.preteyeres.2015.06.002
Molecular pathogenesis of retinal and choroidal vascular diseases
Abstract
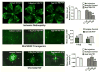
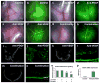
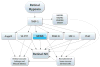
There are two major types of ocular neovascularization that affect the retina, retinal neovascularization (NV) and subretinal or choroidal NV. Retinal NV occurs in a group of diseases referred to as ischemic retinopathies in which damage to retinal vessels results in retinal ischemia. Most prevalent of these are diabetic retinopathy and retinal vein occlusions. Subretinal and choroidal NV occur in diseases of the outer retina and Bruch's membrane, the most prevalent of which is age-related macular degeneration. Numerous studies in mouse models have helped to elucidate the molecular pathogenesis underlying retinal, subretinal, and choroidal NV. There is considerable overlap because the precipitating event in each is stabilization of hypoxia inducible factor-1 (HIF-1) which leads to upregulation of several hypoxia-regulated gene products, including vascular endothelial growth factor (VEGF), angiopoietin 2, vascular endothelial-protein tyrosine phosphatase (VE-PTP), and several others. Stimulation of VEGF signaling and suppression of Tie2 by angiopoietin 2 and VE-PTP are critical for sprouting of retinal, subretinal, and choroidal NV, with perturbation of Bruch's membrane also needed for the latter. Additional HIF-1-regulated gene products cause further stimulation of the NV. It is difficult to model macular edema in animals and therefore proof-of-concept clinical trials were done and demonstrated that VEGF plays a central role and that suppression of Tie2 is also important. Neutralization of VEGF is currently the first line therapy for all of the above disease processes, but new treatments directed at some of the other molecular targets, particularly stabilization of Tie2, are likely to provide additional benefit for subretinal/choroidal NV and macular edema. In addition, the chronicity of these diseases as well as the implication of VEGF as a cause of retinal nonperfusion and progression of background diabetic retinopathy make sustained delivery approaches for VEGF antagonists a priority.
Keywords: Age-related macular degeneration; Angiogenesis; Angiopoietins; Diabetic retinopathy; Hypoxia-inducible factor-1; Platelet-derived growth factor; TIE2; Vascular endothelial growth factor; Vascular endothelial-protein tyrosine phosphatase.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures






References
-
- Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, Ferrara N, King GL, Smith LEH. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA. 1995;92 (23):10457–10461. - PMC - PubMed
-
- Alon T, Hemo I, Itin A, Pe’er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nature Med. 1995;1 (10):1024–1028. - PubMed
-
- Auricchio A, Behling KC, Maguire AM, O’Conner EM, Bennett J, Wilson JM, Tolentino MJ. Inhibition of retinal neovascularization by intraocular viral-mediated delivery of anti-angiogenic agents. Mol Ther. 2002;6 (4):490–494. - PubMed
-
- Avery RL, Castellarin AA, Steinle NC, Dhoot DS, Pieramici DJ, See R, Couvillion S, Nasir MA, Rabena MD, Le K, Maia M, Visich JE. Systemic pharmacokinenetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol. 2014;98 (12):1543–1546. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

