Citrullination of myofilament proteins in heart failure
- PMID: 26113265
- PMCID: PMC4614685
- DOI: 10.1093/cvr/cvv185
Citrullination of myofilament proteins in heart failure
Abstract
Aims: Citrullination, the post-translational conversion of arginine to citrulline by the enzyme family of peptidylarginine deiminases (PADs), is associated with several diseases, and specific citrullinated proteins have been shown to alter function while others act as auto-antigens. In this study, we identified citrullinated proteins in human myocardial samples, from healthy and heart failure patients, and determined several potential functional consequences. Further we investigated PAD isoform cell-specific expression in the heart.
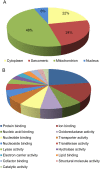
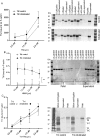
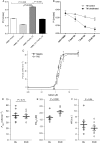
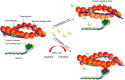
Methods and results: A citrullination-targeted proteomic strategy using data-independent (SWATH) acquisition method was used to identify the modified cardiac proteins. Citrullinated-induced sarcomeric proteins were validated using two-dimensional gel electrophoresis and investigated using biochemical and functional assays. Myocardial PAD isoforms were confirmed by RT-PCR with PAD2 being the major isoform in myocytes. In total, 304 citrullinated sites were identified that map to 145 proteins among the three study groups: normal, ischaemia, and dilated cardiomyopathy. Citrullination of myosin (using HMM fragment) decreased its intrinsic ATPase activity and inhibited the acto-HMM-ATPase activity. Citrullinated TM resulted in stronger F-actin binding and inhibited the acto-HMM-ATPase activity. Citrullinated TnI did not alter the binding to F-actin or acto-HMM-ATPase activity. Overall, citrullination of sarcomeric proteins caused a decrease in Ca(2+) sensitivity in skinned cardiomyocytes, with no change in maximal calcium-activated force or hill coefficient.
Conclusion: Citrullination unique to the cardiac proteome was identified. Our data indicate important structural and functional alterations to the cardiac sarcomere and the contribution of protein citrullination to this process.
Keywords: Citrullination; Heart failure; Myofilament; Peptidylarginine deiminases; Sarcomere.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author 2015. For permissions please email: journals.permissions@oup.com.
Figures



Comment in
-
A sarcomeric protein tongue-twister: post-translation, citrullination/deimination and elimination of arginine residues.Cardiovasc Res. 2015 Nov 1;108(2):212-4. doi: 10.1093/cvr/cvv220. Epub 2015 Sep 20. Cardiovasc Res. 2015. PMID: 26392345 No abstract available.
Similar articles
-
Protein Citrullination by Peptidyl Arginine Deiminase/Arginine Deiminase Homologs in Members of the Human Microbiota and Its Recognition by Anti-Citrullinated Protein Antibodies.Int J Mol Sci. 2024 May 10;25(10):5192. doi: 10.3390/ijms25105192. Int J Mol Sci. 2024. PMID: 38791230 Free PMC article.
-
Peptidylarginine deiminase and protein citrullination in prion diseases: strong evidence of neurodegeneration.Prion. 2013 Jan-Feb;7(1):42-6. doi: 10.4161/pri.22380. Epub 2012 Sep 28. Prion. 2013. PMID: 23022892 Free PMC article. Review.
-
Citrullination is linked to reduced Ca2+ sensitivity in hearts of a murine model of rheumatoid arthritis.Acta Physiol (Oxf). 2022 Nov;236(3):e13869. doi: 10.1111/apha.13869. Epub 2022 Sep 2. Acta Physiol (Oxf). 2022. PMID: 36002394 Free PMC article.
-
Placental Protein Citrullination Signatures Are Modified in Early- and Late-Onset Fetal Growth Restriction.Int J Mol Sci. 2025 Apr 29;26(9):4247. doi: 10.3390/ijms26094247. Int J Mol Sci. 2025. PMID: 40362485 Free PMC article.
-
Peptidylarginine deiminase enzymes and citrullinated proteins in female reproductive physiology and associated diseases†.Biol Reprod. 2022 Dec 10;107(6):1395-1410. doi: 10.1093/biolre/ioac173. Biol Reprod. 2022. PMID: 36087287 Free PMC article. Review.
Cited by
-
Site-specific acetyl-mimetic modification of cardiac troponin I modulates myofilament relaxation and calcium sensitivity.J Mol Cell Cardiol. 2020 Feb;139:135-147. doi: 10.1016/j.yjmcc.2020.01.007. Epub 2020 Jan 22. J Mol Cell Cardiol. 2020. PMID: 31981571 Free PMC article.
-
Anti-modified citrullinated vimentin antibody: a novel biomarker associated with cardiac systolic dysfunction in patients with rheumatoid arthritis.BMC Cardiovasc Disord. 2020 Aug 26;20(1):390. doi: 10.1186/s12872-020-01676-x. BMC Cardiovasc Disord. 2020. PMID: 32847506 Free PMC article.
-
Citrullination as a plausible link to periodontitis, rheumatoid arthritis, atherosclerosis and Alzheimer's disease.J Oral Microbiol. 2018 Jun 22;10(1):1487742. doi: 10.1080/20002297.2018.1487742. eCollection 2018. J Oral Microbiol. 2018. PMID: 29963294 Free PMC article. Review.
-
Replacing voltage sensor arginines with citrulline provides mechanistic insight into charge versus shape.J Gen Physiol. 2018 Jul 2;150(7):1017-1024. doi: 10.1085/jgp.201812075. Epub 2018 Jun 4. J Gen Physiol. 2018. PMID: 29866793 Free PMC article.
-
Cardiac myosin motor deficits are associated with left ventricular dysfunction in human ischemic heart failure.Am J Physiol Heart Circ Physiol. 2023 Feb 1;324(2):H198-H209. doi: 10.1152/ajpheart.00272.2022. Epub 2022 Dec 16. Am J Physiol Heart Circ Physiol. 2023. PMID: 36525480 Free PMC article.
References
-
- Chang X, Han J. Expression of peptidylarginine deiminase type 4 (PAD4) in various tumors. Mol Carcinog 2006;45:183–196. - PubMed
-
- Mastronardi FG, Noor A, Wood DD, Paton T, Moscarello MA. Peptidyl argininedeiminase 2 CpG island in multiple sclerosis white matter is hypomethylated. J Neurosci Res 2007;85:2006–2016. - PubMed
-
- Ishigami A, Ohsawa T, Hiratsuka M, Taguchi H, Kobayashi S, Saito Y, Murayama S, Asaga H, Toda T, Kimura N, Maruyama N. Abnormal accumulation of citrullinated proteins catalyzed by peptidylarginine deiminase in hippocampal extracts from patients with Alzheimer's disease. J Neurosci Res 2005;80:120–128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

