Deregulation of Fas ligand expression as a novel cause of autoimmune lymphoproliferative syndrome-like disease
- PMID: 26113417
- PMCID: PMC4800681
- DOI: 10.3324/haematol.2014.114967
Deregulation of Fas ligand expression as a novel cause of autoimmune lymphoproliferative syndrome-like disease
Abstract
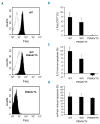
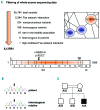
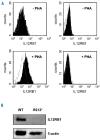
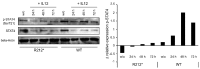
Autoimmune lymphoproliferative syndrome is frequently caused by mutations in genes involved in the Fas death receptor pathway, but for 20-30% of patients the genetic defect is unknown. We observed that treatment of healthy T cells with interleukin-12 induces upregulation of Fas ligand and Fas ligand-dependent apoptosis. Consistently, interleukin-12 could not induce apoptosis in Fas ligand-deficient T cells from patients with autoimmune lymphoproliferative syndrome. We hypothesized that defects in the interleukin-12 signaling pathway may cause a similar phenotype as that caused by mutations of the Fas ligand gene. To test this, we analyzed 20 patients with autoimmune lymphoproliferative syndrome of unknown cause by whole-exome sequencing. We identified a homozygous nonsense mutation (c.698G>A, p.R212*) in the interleukin-12/interleukin-23 receptor-component IL12RB1 in one of these patients. The mutation led to IL12RB1 protein truncation and loss of cell surface expression. Interleukin-12 and -23 signaling was completely abrogated as demonstrated by deficient STAT4 phosphorylation and interferon γ production. Interleukin-12-mediated expression of membrane-bound and soluble Fas ligand was lacking and basal expression was much lower than in healthy controls. The patient presented with the classical symptoms of autoimmune lymphoproliferative syndrome: chronic non-malignant, non-infectious lymphadenopathy, splenomegaly, hepatomegaly, elevated numbers of double-negative T cells, autoimmune cytopenias, and increased levels of vitamin B12 and interleukin-10. Sanger sequencing and whole-exome sequencing excluded the presence of germline or somatic mutations in genes known to be associated with the autoimmune lymphoproliferative syndrome. Our data suggest that deficient regulation of Fas ligand expression by regulators such as the interleukin-12 signaling pathway may be an alternative cause of autoimmune lymphoproliferative syndrome-like disease.
Copyright© Ferrata Storti Foundation.
Figures

References
-
- Canale VC, Smith CH. Chronic lymphadenopathy simulating malignant lymphoma. J Pediatr. 1967;70(6):891–899. - PubMed
-
- Fleisher TA, Oliveira JB. Monogenic defects in lymphocyte apoptosis. Curr Opin Allergy Clin Immunol. 2013;12(6):609–615. - PubMed
-
- Fisher GH, Rosenberg FJ, Straus SE, et al. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995;81(6):935–946. - PubMed
-
- Rieux-Laucat F, Le Deist F, Hivroz C, et al. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268(5215):1347–1349. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

