A kinematic model for 3-D head-free gaze-shifts
- PMID: 26113816
- PMCID: PMC4461827
- DOI: 10.3389/fncom.2015.00072
A kinematic model for 3-D head-free gaze-shifts
Abstract
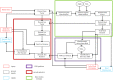
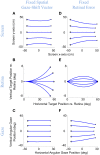
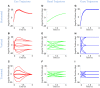
Rotations of the line of sight are mainly implemented by coordinated motion of the eyes and head. Here, we propose a model for the kinematics of three-dimensional (3-D) head-unrestrained gaze-shifts. The model was designed to account for major principles in the known behavior, such as gaze accuracy, spatiotemporal coordination of saccades with vestibulo-ocular reflex (VOR), relative eye and head contributions, the non-commutativity of rotations, and Listing's and Fick constraints for the eyes and head, respectively. The internal design of the model was inspired by known and hypothesized elements of gaze control physiology. Inputs included retinocentric location of the visual target and internal representations of initial 3-D eye and head orientation, whereas outputs were 3-D displacements of eye relative to the head and head relative to shoulder. Internal transformations decomposed the 2-D gaze command into 3-D eye and head commands with the use of three coordinated circuits: (1) a saccade generator, (2) a head rotation generator, (3) a VOR predictor. Simulations illustrate that the model can implement: (1) the correct 3-D reference frame transformations to generate accurate gaze shifts (despite variability in other parameters), (2) the experimentally verified constraints on static eye and head orientations during fixation, and (3) the experimentally observed 3-D trajectories of eye and head motion during gaze-shifts. We then use this model to simulate how 2-D eye-head coordination strategies interact with 3-D constraints to influence 3-D orientations of the eye-in-space, and the implications of this for spatial vision.
Keywords: Listing's law; gaze-shift; head movement; saccade; vestibulo-ocular reflex (VOR).
Figures






Similar articles
-
3-Dimensional eye-head coordination in gaze shifts evoked during stimulation of the lateral intraparietal cortex.Neuroscience. 2009 Dec 15;164(3):1284-302. doi: 10.1016/j.neuroscience.2009.08.066. Epub 2009 Sep 4. Neuroscience. 2009. PMID: 19733631
-
Violations of Listing's law after large eye and head gaze shifts.J Neurophysiol. 1992 Jul;68(1):309-18. doi: 10.1152/jn.1992.68.1.309. J Neurophysiol. 1992. PMID: 1517824
-
Electrical stimulation of the frontal eye fields in the head-free macaque evokes kinematically normal 3D gaze shifts.J Neurophysiol. 2010 Dec;104(6):3462-75. doi: 10.1152/jn.01032.2009. Epub 2010 Sep 29. J Neurophysiol. 2010. PMID: 20881198
-
Neural control of 3-D gaze shifts in the primate.Prog Brain Res. 2003;142:109-24. doi: 10.1016/s0079-6123(03)42009-8. Prog Brain Res. 2003. PMID: 12693257 Review.
-
Listing's law: clinical significance and implications for neural control.Surv Ophthalmol. 2004 Nov-Dec;49(6):563-75. doi: 10.1016/j.survophthal.2004.08.002. Surv Ophthalmol. 2004. PMID: 15530944 Review.
Cited by
-
Eye-head-hand coordination during visually guided reaches in head-unrestrained macaques.J Neurophysiol. 2019 Nov 1;122(5):1946-1961. doi: 10.1152/jn.00072.2019. Epub 2019 Sep 18. J Neurophysiol. 2019. PMID: 31533015 Free PMC article.
-
Gaze-in-wild: A dataset for studying eye and head coordination in everyday activities.Sci Rep. 2020 Feb 13;10(1):2539. doi: 10.1038/s41598-020-59251-5. Sci Rep. 2020. PMID: 32054884 Free PMC article.
-
Eye-Head Coordination in 31 Space Shuttle Astronauts during Visual Target Acquisition.Sci Rep. 2017 Oct 27;7(1):14283. doi: 10.1038/s41598-017-14752-8. Sci Rep. 2017. PMID: 29079792 Free PMC article.
-
Causal Inference for Cross-Modal Action Selection: A Computational Study in a Decision Making Framework.Front Comput Neurosci. 2016 Jun 23;10:62. doi: 10.3389/fncom.2016.00062. eCollection 2016. Front Comput Neurosci. 2016. PMID: 27445780 Free PMC article.
-
Head-neck rotational movements using DidRen laser test indicate children and seniors' lower performance.PLoS One. 2019 Jul 25;14(7):e0219515. doi: 10.1371/journal.pone.0219515. eCollection 2019. PLoS One. 2019. PMID: 31344044 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

