Progressive decline in tacrolimus clearance after renal transplantation is partially explained by decreasing CYP3A4 activity and increasing haematocrit
- PMID: 26114223
- PMCID: PMC4574839
- DOI: 10.1111/bcp.12703
Progressive decline in tacrolimus clearance after renal transplantation is partially explained by decreasing CYP3A4 activity and increasing haematocrit
Abstract
Aims: The long-term disposition of tacrolimus following kidney transplantation is characterized by a gradual decrease in dose requirements and increase in dose-corrected exposure. This phenomenon has been attributed to a progressive decline in cytochrome P450 3A4 (CYP3A4) activity, although this has never been demonstrated in vivo.
Methods: Sixty-five tacrolimus- and 10 cyclosporine-treated renal transplant recipients underwent pharmacokinetic testing at day 7 and months 1, 3, 6 and 12 after transplantation, including 8-h area under the concentration-time curve (AUC) for tacrolimus or cyclosporine and assessment of CYP3A4 activity using oral and intravenous midazolam (MDZ) drug probes.
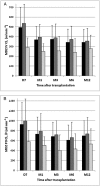
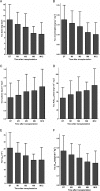
Results: Tacrolimus clearance decreased gradually throughout the entire first year but only in CYP3A5*3/*3 homozygous recipients (25.6 ± 11.1 l h(-1) at day 7; 17 ± 9.1 l h(-1) at month 12; P < 0.001). In mixed model analysis, decreasing CYP3A4 activity, measured by apparent oral MDZ clearance (924 ± 443 ml min(-1) at day 7 vs. 730 ± 344 ml min(-1) at month 1; P < 0.001), explained 55.4% of the decline in tacrolimus clearance in the first month. CYP3A4 activity decreased by 18.9 ml min(-1) for every milligram of methylprednisolone dose tapering within the first month; beyond this point it remained stable. A gradual rise in haematocrit throughout the entire first year explained 31.7% of the decrease in tacrolimus clearance in the first month and 23.6% of the decrease between months 1 and 12. Cyclosporine clearance did not change over time.
Conclusions: The maturation of tacrolimus disposition in the first year after renal transplantation observed in CYP3A5*3/*3 homozygous patients can partly be explained by a (steroid tapering-related) decline in CYP3A4 activity and a progressive increase in haematocrit.
Keywords: CYP3A4; CYP3A5; CYP3A5*1; cyclosporine; kidney transplantation; tacrolimus.
© 2015 The British Pharmacological Society.
Figures
Comment in
-
Response to 'Tacrolimus pharmacokinetics after kidney transplantation--Influence of changes in haematocrit and steroid dose'.Br J Clin Pharmacol. 2015 Dec;80(6):1473-4. doi: 10.1111/bcp.12728. Epub 2015 Sep 15. Br J Clin Pharmacol. 2015. PMID: 26235051 Free PMC article. No abstract available.
-
Tacrolimus pharmacokinetics after kidney transplantation--Influence of changes in haematocrit and steroid dose.Br J Clin Pharmacol. 2015 Dec;80(6):1475-6. doi: 10.1111/bcp.12729. Epub 2015 Sep 15. Br J Clin Pharmacol. 2015. PMID: 26235203 Free PMC article. No abstract available.
References
-
- Schiff J, Cole E, Cantarovich M. Therapeutic monitoring of calcineurin inhibitors for the nephrologist. Clin J Am Soc Nephrol. 2007;2:374–84. - PubMed
-
- De Jonge H, Naesens M, Kuypers DRJ. New insights into the pharmacokinetics and pharmacodynamics of the calcineurin inhibitors and mycophenolic acid: possible consequences for therapeutic drug monitoring in solid organ transplantation. Ther Drug Monit. 2009;31:416–35. - PubMed
-
- Meier-Kriesche HU, Li S, Gruessner RWG, Fung JJ, Bustami RT, Barr ML, Leichtman AB. Immunosuppression: evolution in practice and trends, 1994–2004. Am J Transplant. 2006;6:1111–31. - PubMed
-
- Aoyama T, Yamano S, Waxman DJ, Lapenson DP, Meyer UA, Fischer V, Tyndale R, Inaba T, Kalow W, Gelboin HV, Gonzalez FJ. Cytochrome P-450 hPCN3, a novel cytochrome P-450 IIIA gene product that is differentially expressed in adult human liver. cDNA and deduced amino acid sequence and distinct specificities of cDNA-expressed hPCN1 and hPCN3 for the metabolism of steroid hormones and cyclosporine. J Biol Chem. 1989;264:10388–95. - PubMed
-
- Dai Y, Hebert MF, Isoherranen N, Davis CL, Marsh C, Shen DD, Thummel KE. Effect of CYP3A5 polymorphism on tacrolimus metabolic clearance in vitro. Drug Metab Dispos. 2006;34:836–47. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

