Sensitive detection of lysosomal membrane permeabilization by lysosomal galectin puncta assay
- PMID: 26114578
- PMCID: PMC4590643
- DOI: 10.1080/15548627.2015.1063871
Sensitive detection of lysosomal membrane permeabilization by lysosomal galectin puncta assay
Abstract
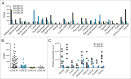
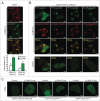
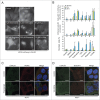
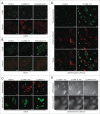
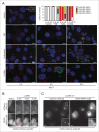
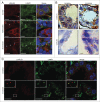
Lysosomal membrane permeabilization (LMP) contributes to tissue involution, degenerative diseases, and cancer therapy. Its investigation has, however, been hindered by the lack of sensitive methods. Here, we characterize and validate the detection of galectin puncta at leaky lysosomes as a highly sensitive and easily manageable assay for LMP. LGALS1/galectin-1 and LGALS3/galectin-3 are best suited for this purpose due to their widespread expression, rapid translocation to leaky lysosomes and availability of high-affinity antibodies. Galectin staining marks individual leaky lysosomes early during lysosomal cell death and is useful when defining whether LMP is a primary or secondary cause of cell death. This sensitive method also reveals that cells can survive limited LMP and confirms a rapid formation of autophagic structures at the site of galectin puncta. Importantly, galectin staining detects individual leaky lysosomes also in paraffin-embedded tissues allowing us to demonstrate LMP in tumor xenografts in mice treated with cationic amphiphilic drugs and to identify a subpopulation of lysosomes that initiates LMP in involuting mouse mammary gland. The use of ectopic fluorescent galectins renders the galectin puncta assay suitable for automated screening and visualization of LMP in live cells and animals. Thus, the lysosomal galectin puncta assay opens up new possibilities to study LMP in cell death and its role in other cellular processes such as autophagy, senescence, aging, and inflammation.
Keywords: C. elegans; breast involution; cell death; galectin; lysosome.
Figures


References
-
- Kroemer G, Jäättelä M. Lysosomes and autophagy in cell death control. Nat Rev Cancer 2005; 5:886-97; PMID:16239905; http://dx.doi.org/10.1038/nrc1738 - DOI - PubMed
-
- Aits S, Jäättelä M. Lysosomal cell death at a glance. J Cell Sci 2013; 126:1905-12; PMID:23720375; http://dx.doi.org/10.1242/jcs.091181 - DOI - PubMed
-
- Cesen MH, Pegan K, Spes A, Turk B. Lysosomal pathways to cell death and their therapeutic applications. Exp Cell Res 2012; 318:1245-51; PMID:22465226; http://dx.doi.org/10.1016/j.yexcr.2012.03.005 - DOI - PubMed
-
- Appelqvist H, Waster P, Kagedal K, Ollinger K. The lysosome: from waste bag to potential therapeutic target. J Mole Cell biol 2013; 5:214-26; PMID: 23918283; http://dx.doi.org/10.1093/jmcb/mjt022 - DOI - PubMed
-
- Boya P, Kroemer G. Lysosomal membrane permeabilization in cell death. Oncogene 2008; 27:6434-51.; PMID:18955971; http://dx.doi.org/10.1038/onc.2008.310 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
