Chelation for autism spectrum disorder (ASD)
- PMID: 26114777
- PMCID: PMC6457964
- DOI: 10.1002/14651858.CD010766.pub2
Chelation for autism spectrum disorder (ASD)
Abstract
Background: It has been suggested that the severity of autism spectrum disorder (ASD) symptoms is positively correlated with the level of circulating or stored toxic metals, and that excretion of these heavy metals, brought about by the use of pharmaceutical chelating agents, results in improved symptoms.
Objectives: To assess the potential benefits and adverse effects of pharmaceutical chelating agents (referred to as chelation therapy throughout this review) for autism spectrum disorder (ASD) symptoms.
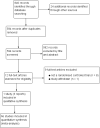
Search methods: We searched the following databases on 6 November 2014: CENTRAL, Ovid MEDLINE, Ovid MEDLINE In-Process, Embase, PsycINFO, Cumulative Index to Nursing and Allied Health Literature (CINAHL) and 15 other databases, including three trials registers. In addition we checked references lists and contacted experts.
Selection criteria: All randomised controlled trials of pharmaceutical chelating agents compared with placebo in individuals with ASD.
Data collection and analysis: Two review authors independently selected studies, assessed them for risk of bias and extracted relevant data. We did not conduct a meta-analysis, as only one study was included.
Main results: We excluded nine studies because they were non-randomised trials or were withdrawn before enrolment. We included one study, which was conducted in two phases. During the first phase of the study, 77 children with ASD were randomly assigned to receive seven days of glutathione lotion or placebo lotion, followed by three days of oral dimercaptosuccinic acid (DMSA). Forty-nine children who were found to be high excreters of heavy metals during phase one continued on to phase two to receive three days of oral DMSA or placebo followed by 11 days off, with the cycle repeated up to six times. The second phase thus assessed the effectiveness of multiple doses of oral DMSA compared with placebo in children who were high excreters of heavy metals and who received a three-day course of oral DMSA. Overall, no evidence suggests that multiple rounds of oral DMSA had an effect on ASD symptoms.
Authors' conclusions: This review included data from only one study, which had methodological limitations. As such, no clinical trial evidence was found to suggest that pharmaceutical chelation is an effective intervention for ASD. Given prior reports of serious adverse events, such as hypocalcaemia, renal impairment and reported death, the risks of using chelation for ASD currently outweigh proven benefits. Before further trials are conducted, evidence that supports a causal link between heavy metals and autism and methods that ensure the safety of participants are needed.
Conflict of interest statement
Stephen James is employed by Southwest Autism Research and Resource Center. His work is not related to chelation, and he receives no personal funds.
Shawn W Stevenson was employed by Autism Victoria at the time of this review. He was an unpaid Board Member of Support and Advocacy for Autism Spectrum Individuals and Families. Shawn is currently employed by the University of Melbourne and has received no personal funds.
Natalie Silove works at the Children's Hospital Westmead, which is enrolling up to eight participants in a phase two drug trial in adolescents with fragile X syndrome. It is not related to autism or to chelation. Natalie receives no personal funds.
Katrina Williams gave a talk about treatments for autism at a symposium organised by Janssen‐Cilag Pty Ltd. Janssen‐Cilag had no control over the contents of the talk, and the speaker's fee was paid to the University that employs her. She does not have an ongoing relationship with Janssen‐Cilag. She is not involved in any funded work relevant to chelation therapy.
Figures
Update of
- doi: 10.1002/14651858.CD010766
References
References to studies included in this review
Adams 2009 {published data only}
-
- NCT00811083. Dimercaptosuccinic acid (DMSA) treatment of children with autism and heavy metal toxicity. http://clinicaltrials.gov/show/NCT00811083 (accessed 19 December 2013).
References to studies excluded from this review
Blaucok‐Busch 2012 {published data only}
Eppright 1996 {published data only}
-
- Eppright TD, Sanfacon JA, Horwitz EA. Attention deficit hyperactivity disorder, infantile autism, and elevated blood‐lead: a possible relationship. Missouri Medicine 1996;93(3):136‐8. - PubMed
Geier 2006a {published data only}
-
- Geier DA, Geier MR. A clinical trial of combined anti‐androgen and anti‐heavy metal therapy in autistic disorders. Neuro Endocrinology Letters 2006;27(6):833‐8. - PubMed
Geier 2006b {published data only}
-
- Geier DA, Geier MR. A prospective assessment of porphyrins in autistic disorders: a potential marker for heavy metal exposure. Neurotoxicity Research 2006;10(1):57‐64. - PubMed
Geier 2007 {published data only}
-
- Geier DA, Geier MR. A prospective study of mercury toxicity biomarkers in autistic spectrum disorders. Journal of Toxicology and Environmental Health, Part A 2007;70(20):1723‐30. - PubMed
Lonsdale 2002 {published data only}
-
- Lonsdale D, Shamberger RJ, Audhya T. Treatment of autism spectrum children with thiamine tetrahydrofurfuryl disulfide: a pilot study. Neuro Endocrinology Letters 2002;23(4):303‐8. - PubMed
Nataf 2006 {published data only}
-
- Nataf R, Skorupka C, Amet L, Lam A, Springbett A, Lathe R. Porphyrinuria in childhood autistic disorder: implications for environmental toxicity. Toxicology and Applied Pharmacology 2006;214(2):99‐108. - PubMed
NCT00376194 {published data only}
-
- NCT00376194. Mercury chelation to treat autism. http://clinicaltrials.gov/show/NCT00376194 (accessed 19 December 2013).
Patel 2007 {published data only}
-
- Patel K, Curtis LT. A comprehensive approach to treating autism and attention‐deficit hyperactivity disorder: a prepilot study. Journal of Alternative and Complementary Medicine 2007;13(10):1091‐7. - PubMed
Additional references
Adams 2013
-
- Adams JB, Audhya T, McDonough‐Means S, Rubin RA, Quig D, Geis E, et al. Toxicological status of children with autism vs. neurotypical children and the association with autism severity. Biological Trace Element Research 2013;151(2):171‐80. - PubMed
Albizzati 2012
-
- Albizzati A, More L, Candia D, Saccani M, Lenti C. Normal concentrations of heavy metals in autistic spectrum disorders. Minerva Pediatrica 2012;64(1):27‐31. - PubMed
Amin‐Zaki 1974
-
- Amin‐Zaki L, Elhassani S, Majeed MA, Clarkson TW, Doherty RA, Greenwood M. Intra‐uterine methylmercury poisoning in Iraq. Pediatrics 1974;54(5):587‐95. - PubMed
APA 2013
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐5). 5th Edition. Arlington, VA: American Psychiatric Publishing, 2013.
Aschner 1990
-
- Aschner M, Aschner JL. Mercury neurotoxicity: mechanisms of blood‐brain barrier transport. Neuroscience and Biobehavioral Reviews 1990;14(2):169‐76. - PubMed
Bakir 1973
-
- Bakir F, Damluji SF, Amin‐Zaki L, Murtadha M, Khalidi A, Al‐Rawi NY, et al. Methylmercury poisoning in Iraq. Science 1973;181(4096):230‐41. - PubMed
Bernard 2001
-
- Bernard S, Enayati A, Redwood L, Roger H, Binstock T. Autism: a novel form of mercury poisoning. Medical Hypotheses 2001;56(4):462‐71. - PubMed
Bradstreet 2003
-
- Bradstreet J, Geier DA, Kartzinel JJ, Adams JB, Geier MR. A case‐control study of mercury burden in children with autistic spectrum disorders. Journal of American Physicians and Surgeons 2003;8(3):76‐9.
Brown 2006
-
- Brown MJ, Willis T, Omalu B, Leiker R. Deaths resulting from hypocalcemia after administration of edetate disodium: 2003‐2005. Pediatrics 2006;118(2):e534‐6. - PubMed
Cheuk 2011
Christon 2010
-
- Christon LM, Mackintosh VH, Myers BJ. Use of complementary and alternative medicine (CAM) treatments by parents of children with autism spectrum disorders. Research in Autism Spectrum Disorders 2010;4(2):249‐59.
Cohen 1976
-
- Cohen DJ, Johnson WT, Caparulo BK. Pica and elevated blood lead level in autistic and atypical children. American Journal of Diseases of Children 1976;130(1):47‐8. - PubMed
Cohen 1982
-
- Cohen DJ, Paul R, Anderson GM, Harcherik DF. Blood lead in autistic children. Lancet 1982;320(8289):94‐5. - PubMed
Counter 2004
-
- Counter SA, Buchanan LH. Mercury exposure in children: a review. Toxicology and Applied Pharmacology 2004;198(2):209‐30. - PubMed
Dans 2002
Davis 2013
-
- Davis TN, O'Reilly M, Kang S, Lang R, Rispoli M, Sigafoos J, et al. Chelation treatment for autism spectrum disorders: a systematic review. Research in Autism Spectrum Disorders 2013;7(1):49‐55.
Deth 2008
-
- Deth R, Muratore C, Benzecry J, Power‐Charnitsky V, Waly M. How environmental and genetic factors combine to cause autism: a redox/methylation hypothesis. Neurotoxicology 2008;29(1):190‐201. - PubMed
Drugs.com 2012
-
- Drugs.com. Drug information online. www.drugs.com/drug‐class/chelating‐agents.html (accessed 10 January 2012).
Egger 1997
Ernst 2000
-
- Ernst E. Chelation therapy for coronary heart disease: an overview of all clinical investigations. American Heart Journal 2000;140(1):139‐41. - PubMed
Garrecht 2011
Geretsegger 2014
Goin‐Kochel 2009
-
- Goin‐Kochel RP, Mackintosh VH, Myers BJ. Parental reports on the efficacy of treatments and therapies for their children with autism spectrum disorders. Research in Autism Spectrum Disorders 2009;3(2):528‐37.
Goldman 2001
-
- Goldman LR, Shannon MW, American Academy of Pediatrics: Committee on Environmental Health. Technical report: mercury in the environment: implications for pediatricians. Pediatrics 2001;108(1):197‐205. - PubMed
Golnik 2009
-
- Golnik AE, Ireland M. Complementary alternative medicine for children with autism: a physician survey. Journal of Autism and Developmental Disorders 2009;39(7):996‐1005. - PubMed
Grandjean 1997
-
- Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, et al. Cognitive deficit in 7‐year‐old children with prenatal exposure to methylmercury. Neurotoxicology and Teratology 1997;19(6):417‐28. - PubMed
Green 2006
-
- Green VA, Pituch KA, Itchon J, Choi A, O'Reilly M, Sigafoos J. Internet survey of treatments used by parents of children with autism. Research in Developmental Disabilities 2006;27(1):70‐84. - PubMed
Hallmayer 2011
Hanson 2007
-
- Hanson E, Kalish L, Bunce E, Curtis C, McDaniel S, Ware J, et al. Use of complementary and alternative medicine among children diagnosed with autism spectrum disorder. Journal of Autism and Developmental Disorders 2007;37(4):629‐36. - PubMed
Hedge 2009
Hertz‐Picciotto 2010
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holmes 2003
-
- Holmes AS, Blaxill MF, Haley BE. Reduced levels of mercury in first baby haircuts of autistic children. International Journal of Toxicology 2003;22(4):277‐85. - PubMed
James 2011
Kern 2007
-
- Kern JK, Grannemann BD, Trivedi MH, Adams JB. Sulfhydryl‐reactive metals in autism. Journal of Toxicology and Environmental Health, Part A 2007;70(8):715‐21. - PubMed
Kosnett 2010
-
- Kosnett MJ. Chelation for heavy metals (arsenic, lead, and mercury): protective or perilous?. Clinical Pharmacology & Therapeutics 2010;88(3):412‐5. - PubMed
Lecavalier 2006
-
- Lecavalier L. Behavioral and emotional problems in young people with pervasive developmental disorders: relative prevalence, effects of subject characteristics, and empirical classification. Journal of Autism and Developmental Disorders 2006;36(8):1101‐14. - PubMed
Levy 2003
-
- Levy SE, Hyman SL. Use of complementary and alternative treatments for children with autistic spectrum disorders is increasing. Pediatric Annals 2003;32(10):685‐91. - PubMed
Liu 2008
-
- Liu J, Goyer RA, Waalkes MP. Toxic effects of metals. In: Klaassen CD editor(s). Casarett & Doull's Toxicology: The Basic Science of Poisons. 7th Edition. New York: McGraw‐Hill, 2008:931‐79.
Millward 2008
Mitka 2008
-
- Mitka M. Chelation therapy trials halted. JAMA 2008;300(19):2236. - PubMed
Morgan 2002
-
- Morgan BW, Kori S, Thomas JD. Adverse effects in 5 patients receiving EDTA at an outpatient chelation clinic. Veterinary and Human Toxicology 2002;44(5):274‐6. - PubMed
Nye 2005
Osterloh 1986
-
- Osterloh J, Becker CE. Pharmacokinetics of CaNa2EDTA and chelation of lead in renal failure. Clinical Pharmacology and Therapeutics 1986;40(6):686‐93. - PubMed
Rahbar 2013
Semple 2011
-
- Semple S, Hewton C, Paterson F, Angley M. Complementary medicine products used in autism ‐ evidence for efficacy and safety. In: Williams T editor(s). Autism Spectrum Disorders ‐ From Genes to Environment. Rijeka, Croatia: InTech, 2011:77‐100.
Shattuck 2007
Sinha 2006
Sinha 2011
Vamnes 2000
-
- Vamnes JS, Eide R, Isrenn R, Hol PJ, Gjerdet NR. Diagnostic value of a chelating agent in patients with symptoms allegedly caused by amalgam fillings. Journal of Dental Research 2000;79(3):868‐74. - PubMed
Zecavati 2009
-
- Zecavati N, Spence SJ. Neurometabolic disorders and dysfunction in autism spectrum disorders. Current Neurology and Neuroscience Reports 2009;9(2):129‐36. - PubMed
References to other published versions of this review
James 2013
-
- James S, Williams K, Silove N, Stevenson SW. Chelation for autism spectrum disorder (ASD). Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD010766] - DOI
LinkOut - more resources
Full Text Sources
Research Materials