Characterization of Endothelial Progenitor Cell Interactions with Human Tropoelastin
- PMID: 26115013
- PMCID: PMC4482626
- DOI: 10.1371/journal.pone.0131101
Characterization of Endothelial Progenitor Cell Interactions with Human Tropoelastin
Abstract
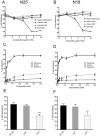
The deployment of endovascular implants such as stents in the treatment of cardiovascular disease damages the vascular endothelium, increasing the risk of thrombosis and promoting neointimal hyperplasia. The rapid restoration of a functional endothelium is known to reduce these complications. Circulating endothelial progenitor cells (EPCs) are increasingly recognized as important contributors to device re-endothelialization. Extracellular matrix proteins prominent in the vessel wall may enhance EPC-directed re-endothelialization. We examined attachment, spreading and proliferation on recombinant human tropoelastin (rhTE) and investigated the mechanism and site of interaction. EPCs attached and spread on rhTE in a dose dependent manner, reaching a maximal level of 56±3% and 54±3%, respectively. EPC proliferation on rhTE was comparable to vitronectin, fibronectin and collagen. EDTA, but not heparan sulfate or lactose, reduced EPC attachment by 81±3%, while full attachment was recovered after add-back of manganese, inferring a classical integrin-mediated interaction. Integrin αVβ3 blocking antibodies decreased EPC adhesion and spreading on rhTE by 39±3% and 56±10% respectively, demonstrating a large contribution from this specific integrin. Attachment of EPCs on N-terminal rhTE constructs N25 and N18 accounted for most of this interaction, accompanied by comparable spreading. In contrast, attachment and spreading on N10 was negligible. αVβ3 blocking antibodies reduced EPC spreading on both N25 and N18 by 45±4% and 42±14%, respectively. In conclusion, rhTE supports EPC binding via an integrin mechanism involving αVβ3. N25 and N18, but not N10 constructs of rhTE contribute to EPC binding. The regulation of EPC activity by rhTE may have implications for modulation of the vascular biocompatibility of endovascular implants.
Conflict of interest statement
Figures

Similar articles
-
A cell adhesive peptide from tropoelastin promotes sequential cell attachment and spreading via distinct receptors.FEBS J. 2017 Jul;284(14):2216-2230. doi: 10.1111/febs.14114. Epub 2017 Jun 15. FEBS J. 2017. PMID: 28544621
-
Blood pressure control is not enough to normalize endothelial repair by progenitor cells.Am J Physiol Heart Circ Physiol. 2020 Oct 1;319(4):H744-H752. doi: 10.1152/ajpheart.00333.2020. Epub 2020 Aug 14. Am J Physiol Heart Circ Physiol. 2020. PMID: 32795193
-
Stent coated with antibody against vascular endothelial-cadherin captures endothelial progenitor cells, accelerates re-endothelialization, and reduces neointimal formation.Arterioscler Thromb Vasc Biol. 2011 Dec;31(12):2798-805. doi: 10.1161/ATVBAHA.111.226134. Epub 2011 Oct 20. Arterioscler Thromb Vasc Biol. 2011. PMID: 22015656
-
Endothelial progenitor cells and cardiovascular disease.J Stem Cells. 2014;9(2):93-106. J Stem Cells. 2014. PMID: 25158158 Review.
-
Factors Affecting the Re-Endothelialization of Endothelial Progenitor Cell.DNA Cell Biol. 2021 Jul;40(7):1009-1025. doi: 10.1089/dna.2021.0082. Epub 2021 May 28. DNA Cell Biol. 2021. PMID: 34061680 Review.
Cited by
-
Elastomers in vascular tissue engineering.Curr Opin Biotechnol. 2016 Aug;40:149-154. doi: 10.1016/j.copbio.2016.04.008. Epub 2016 May 2. Curr Opin Biotechnol. 2016. PMID: 27149017 Free PMC article. Review.
-
Small Caliber Compliant Vascular Grafts Based on Elastin-Like Recombinamers for in situ Tissue Engineering.Front Bioeng Biotechnol. 2019 Nov 19;7:340. doi: 10.3389/fbioe.2019.00340. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31803735 Free PMC article.
-
Plasma processing of PDMS based spinal implants for covalent protein immobilization, cell attachment and spreading.J Mater Sci Mater Med. 2018 Nov 30;29(12):178. doi: 10.1007/s10856-018-6181-y. J Mater Sci Mater Med. 2018. PMID: 30506173
-
Vascular endothelial growth factor modified macrophages transdifferentiate into endothelial-like cells and decrease foam cell formation.Biosci Rep. 2017 Jun 21;37(3):BSR20170002. doi: 10.1042/BSR20170002. Print 2017 Jun 30. Biosci Rep. 2017. PMID: 28536311 Free PMC article.
-
Tropoelastin enhances nitric oxide production by endothelial cells.Nanomedicine (Lond). 2016 Jun;11(12):1591-7. doi: 10.2217/nnm-2016-0052. Epub 2016 May 13. Nanomedicine (Lond). 2016. PMID: 27175893 Free PMC article.
References
-
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275: 964–967. - PubMed
-
- Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, et al. Circulating Endothelial Progenitor Cells and Cardiovascular Outcomes. N Engl J Med. 2005;353: 999–1007. - PubMed
-
- Gunsilius E. Evidence from a leukemia model for maintenance of vascular endothelium by bone-marrow-derived endothelial cells. Adv Exp Med Biol. 2003;522: 17–24. - PubMed
-
- Crosby JR, Kaminski WE, Schatteman G, Martin PJ, Raines EW, Seifert RA, et al. Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation. Circ Res. 2000;87: 728–730. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

