Immediate postpartum insertion of intrauterine device for contraception
- PMID: 26115018
- PMCID: PMC10777269
- DOI: 10.1002/14651858.CD003036.pub3
Immediate postpartum insertion of intrauterine device for contraception
Abstract
Background: Women who want to start intrauterine contraception (IUC) during the postpartum period might benefit from IUC insertion immediately after delivery. Postplacental insertion greatly reduces the risk of subsequent pregnancy and eliminates the need for a return visit to start contraception. Without the option of immediate insertion, many women may never return for services or may adopt less effective contraception.
Objectives: Our aim was to examine the outcomes of IUC insertion immediately after placenta delivery (within 10 minutes), especially when compared with insertion at other postpartum times. We focused on successful IUC placement (insertion), subsequent expulsion, and method use.
Search methods: We searched for trials until 1 April 2015. Sources included PubMed (MEDLINE), the Cochrane Central Register of Controlled Trials (CENTRAL), POPLINE, Web of Science, EMBASE, LILACS, ClinicalTrials.gov, and ICTRP. For the original review, the authors contacted investigators to identify other trials.
Selection criteria: We sought randomized controlled trials (RCTs) with at least one treatment arm that involved immediate IUC placement (i.e., within 10 minutes of placenta delivery). Comparison arms could have included early postpartum insertion (from 10 minutes postplacental to hospital discharge) or standard insertion (during a postpartum visit). Trials could also have compared different IUC methods or insertion techniques. Delivery may have been vaginal or cesarean. Primary outcomes were placement (insertion), subsequent expulsion, and method use at study assessment.
Data collection and analysis: For dichotomous outcomes, we used the Mantel-Haenszel odds ratio (OR) with 95% confidence interval (CI). Earlier studies primarily reported results as life-table rates. We aggregated trials in a meta-analysis if they had similar interventions and outcome measures. A sensitivity analysis included studies with moderate or high quality evidence and sufficient outcome data.
Main results: We included 15 trials. Seven studies reported from 2010 to 2014 were added to eight from the original 2001 review. Newer trials compared immediate postplacental insertion versus early (10 minutes to 48 hours) or standard insertion (during the postpartum visit). Of four with full reports, three were small trials. The other three studies had conference abstracts. The eight early trials examined immediate insertion of different devices or insertion techniques. Most studies were published in the 1980s, some with limited reporting.Our sensitivity analysis included trials with sufficient outcome data and moderate or high quality evidence. Four newer trials comparing insertion times met the inclusion criteria. Two studies used the levonorgestrel-releasing intrauterine system (LNG-IUS) after vaginal delivery. The other two trials placed IUC after cesarean section; one used the CuT 380A intrauterine device (IUD) and the other used the LNG-IUS.A pilot trial compared immediate insertion versus early or standard insertion. In groups comparing immediate versus early insertion (N = 30), all women had the LNG-IUS inserted. By six months, the groups had the same expulsion rate and did not differ significantly in IUC use.For immediate versus standard insertion, we conducted meta-analyses of four trials. Insertion rates did not differ significantly between study arms. However, the trial from Uganda showed insertion was more likely for the immediate group, although the estimate was imprecise. In the meta-analysis, expulsion by six months was more likely for the immediate group, but the confidence interval was wide (OR 4.89, 95% CI 1.47 to 16.32; participants = 210; studies = 4). IUC use at six months was more likely with immediate insertion than with standard insertion (OR 2.04, 95% CI 1.01 to 4.09; participants = 243; studies = 4). Study arms did not differ in use at 3 or 12 months in individual small trials.
Authors' conclusions: Recent trials compared different insertion times after vaginal or cesarean delivery. Evidence was limited because studies with full reports generally had small sample sizes. Overall, the quality of evidence was moderate; abstracts and older studies had limited reporting. Ongoing trials will add to the evidence, although some are small. Trials of adequate power are needed to estimate expulsion rates and side effects.The benefit of effective contraception immediately after delivery may outweigh the disadvantage of increased risk for expulsion. Frequent prenatal visits during the third trimester provide the opportunity to discuss effective contraceptive methods and desired timing for initiation. Clinical follow-up can help detect early expulsion, as can educating women about expulsion signs and symptoms.
Conflict of interest statement
Family Health International, now known as FHI 360, conducted two trials (Cole 1984: Kisnisci 1985). An employee of FHI 360 was involved in two trials (Lavin 1983; Apelo 1985). Authors of this review are employed at FHI 360, but none were involved in those trials.
Figures
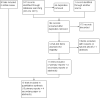















Update of
-
Immediate post-partum insertion of intrauterine devices.Cochrane Database Syst Rev. 2010 May 12;(5):CD003036. doi: 10.1002/14651858.CD003036.pub2. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2015 Jun 26;(6):CD003036. doi: 10.1002/14651858.CD003036.pub3. PMID: 20464722 Updated.
Similar articles
-
Immediate versus delayed postpartum insertion of contraceptive implant and IUD for contraception.Cochrane Database Syst Rev. 2022 Oct 27;10(10):CD011913. doi: 10.1002/14651858.CD011913.pub3. Cochrane Database Syst Rev. 2022. PMID: 36302159 Free PMC article.
-
Interventions for pain with intrauterine device insertion.Cochrane Database Syst Rev. 2015 Jul 29;2015(7):CD007373. doi: 10.1002/14651858.CD007373.pub3. Cochrane Database Syst Rev. 2015. PMID: 26222246 Free PMC article.
-
Hormonal and intrauterine methods for contraception for women aged 25 years and younger.Cochrane Database Syst Rev. 2015 Aug 17;2015(8):CD009805. doi: 10.1002/14651858.CD009805.pub3. Cochrane Database Syst Rev. 2015. PMID: 26280888 Free PMC article.
-
Education for contraceptive use by women after childbirth.Cochrane Database Syst Rev. 2015 Jul 29;2015(7):CD001863. doi: 10.1002/14651858.CD001863.pub4. Cochrane Database Syst Rev. 2015. PMID: 26222129 Free PMC article.
-
Combined hormonal versus nonhormonal versus progestin-only contraception in lactation.Cochrane Database Syst Rev. 2015 Mar 20;2015(3):CD003988. doi: 10.1002/14651858.CD003988.pub2. Cochrane Database Syst Rev. 2015. PMID: 25793657 Free PMC article.
Cited by
-
Patient acceptability, continuation and complication rates with immediate postpartum levonorgestrel intrauterine device insertion at caesarean section and vaginal birth.Aust N Z J Obstet Gynaecol. 2022 Oct;62(5):773-778. doi: 10.1111/ajo.13535. Epub 2022 Apr 21. Aust N Z J Obstet Gynaecol. 2022. PMID: 35451065 Free PMC article.
-
Immediate Postpartum Insertion of Copper Intrauterine Device in a Brazilian University Hospital: Expulsion and Continuation Rates.Rev Bras Ginecol Obstet. 2023 Jan;45(1):31-37. doi: 10.1055/s-0042-1759628. Epub 2023 Mar 6. Rev Bras Ginecol Obstet. 2023. PMID: 36878250 Free PMC article.
-
Acceptance, utilization, and factors associated with immediate postpartum intrauterine contraceptive device among mothers delivered at public health facilities in Hawassa city, Ethiopia: Institution-based study.Reprod Health. 2023 Mar 8;20(1):39. doi: 10.1186/s12978-023-01586-z. Reprod Health. 2023. PMID: 36890509 Free PMC article.
-
Comparison of an additional early visit to routine postpartum care on initiation of long-acting reversible contraception: A randomized trial.Contraception. 2018 Sep;98(3):223-227. doi: 10.1016/j.contraception.2018.05.010. Epub 2018 May 18. Contraception. 2018. PMID: 29778586 Free PMC article. Clinical Trial.
-
Couples' decision-making on post-partum family planning and antenatal counselling in Uganda: A qualitative study.PLoS One. 2021 May 5;16(5):e0251190. doi: 10.1371/journal.pone.0251190. eCollection 2021. PLoS One. 2021. PMID: 33951104 Free PMC article.
References
References to studies included in this review
Ahuja 2014 {published data only (unpublished sought but not used)}
-
- Ahuja R, Rahtore A. Continuation rates of postpartum intrauterine contraceptive device (IUCD) insertion: randomised trial of post placental versus immediate postpartum insertion (conference abstract). BJOG: An International Journal of Obstetrics and Gynaecology 2014;121(Suppl s2):1‐2.
-
- Rathore AM, Ahuja R. Continuation Rates of Immediate Post partum CuT Insertion: Randomized Trial of Post Placental versus Early Post Partum Insertion. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=4103 (accessed 19 August 2014). [CTRI/2012/04/002543]
Apelo 1985 {published and unpublished data}
-
- Apelo RA, Waszak CS. Postpartum IUD insertions in Manila, Philippines. Advances in Contraception 1985;1(4):319‐28. - PubMed
Chen 2010 {published data only (unpublished sought but not used)}
-
- Chen BA, Hayes JL, Hohmann HL, Perriera LK, Reeves MF, Creinin MD. A randomized trial of postplacental compared to delayed insertion of the levonorgestrel‐releasing intrauterine device after vaginal delivery (abstract). Contraception 2009;80(2):205.
Cole 1984 {published and unpublished data}
-
- Cole LP, Edelman DA, Potts DM, Wheler RG, Laufe LE. Postpartum insertion of modified intrauterine devices. Journal of Reproductive Medicine 1984;29(9):677‐82. - PubMed
Dahlke 2011 {published data only}
-
- Dahlke JD, Terpstra ER, Ramseyer AM, Busch JM, Rieg T, Magann EF. Postpartum insertion of levonorgestrel‐‐intrauterine system at three time periods: a prospective randomized pilot study. Contraception 2011;84(3):244‐8. - PubMed
Kisnisci 1985 {published and unpublished data}
-
- Kisnisci H, Champion CB. A study of Delta intrauterine devices in Ankara, Turkey. International Journal of Gynaecology and Obstetrics 1985;23(1):51‐4. - PubMed
Lavin 1983 {published and unpublished data}
-
- Lavin P, Bravo C, Waszak C. Comparison of TCu 200 and Progestasert IUDs. Contraceptive Delivery Systems 1983;4(2):143‐7. - PubMed
Lester 2015 {published data only (unpublished sought but not used)}
-
- Averbach S, Lester F, Fortin J, Byamugisha J, Goldberg A, Kakaire O. Acceptability of the IUD among women who opted out of a randomized controlled trial of intracesarean insertion of the copper‐T 380A in Kampala, Uganda. Contraception 2012;86:318.
-
- Lester F, Kakaire O, Byamugisha J, Averbach S, Fortin J, Maurer R, et al. Intracesarean insertion of the Copper T380A versus 6 weeks post‐cesarean: a randomized clinical trial. Contraception 2015;91(3):198‐203. - PubMed
-
- Lester F, Kakaire O, Byamugisha J, Goldberg A. Intracesarean insertion of the Copper T 380A vs. 6 week post‐cesarean insertion: A pilot randomized controlled trial. 2011 International Conference on Family Planning. 2011 Nov 29‐Dec 01; Dakar, Senegal. www.conftool.com/fpconference2011/index.php?page=browseSessions&form..., (accessed 19 August 2014).
Ogburn 2013 {published data only (unpublished sought but not used)}
-
- Ogburn T, Espey E, Leeman L, Singh R, Pereda B, Carr S. A randomized trial of immediate postpartum versus interval insertion of an intrauterine device. Contraception 2013;88(3):455.
Singh 2014 {published data only (unpublished sought but not used)}
-
- Singh N, Singh S, Pathak A. Comparison of immediate postplacental and early postpartum intrauterine device insertion (abstract). BJOG ‐an International Journal of Obstetrics and Gynaecology 2014;121(Suppl s2):7‐8.
-
- Singh N, Singh S, Singh S, Pathak A. Comparison of immediate post‐placental and early postpartum intra‐uterine device insertion (conference poster). RCOG World Congress 2014; 2014 March 28‐30; Hyderabad (India). ePostersOnline.
Thiery 1980 {published data only}
-
- Thiery M, Pas H, Delbeke L, Kets H. Comparative performance of two copper‐wired IUDs (ML Cu 250 and T Cu 200). Immediate postpartum and interval insertion. Contraceptive Delivery Systems 1980;1(1):27‐35. - PubMed
Van Kets 1987 {published data only}
-
- Kets H, Thiery M, Pas H, Parewijck W. Immediate postpartum insertion: performance of the Nova‐T‐PP and randomized comparison with the Nova‐T. Advances in Contraception 1987;3(1):63‐9. - PubMed
Whitaker 2014 {published data only}
-
- Whitaker AK, Endres LK, Mistretta SQ, Gilliam ML. Postplacental insertion of the levonorgestrel intrauterine device after cesarean delivery vs. delayed insertion: a randomized controlled trial. Contraception 2014;89(6):534‐9. - PubMed
WHO 1980 {published and unpublished data}
-
- World Health Organization. Comparative multicentre trial of three IUDs inserted immediately following delivery of the placenta. Contraception 1980;22(1):9‐18. - PubMed
Xu 1996 {published and unpublished data}
-
- Xu J‐X, Rivera R, Dunson TR, Zhuang L‐Q, Yang X‐L, Ma G‐T, et al. A comparative study of two techniques used in immediate postplacental insertion (IPPI) of the Copper T 380A IUD in Shanghai, People's Republic of China. Contraception 1996;54(1):33‐8. - PubMed
References to studies excluded from this review
Chi 1985 {published data only}
-
- Chi I‐C, Wilkens L, Rogers S. Expulsions in immediate postpartum insertions of Lippes Loop D and Copper T IUDs and their counterpart delta devices ‐‐ an epidemiological analysis. Contraception 1985;32(2):119‐34. - PubMed
Elsedeek 2012 {published data only}
-
- Elsedeek MS. Puerperal and menstrual bleeding patterns with different types of contraceptive device fitted during elective cesarean delivery. International Journal of Gynaecology and Obstetrics 2012;116:31‐4. - PubMed
Eroglu 2006 {published data only}
-
- Eroglu K, Akkuzu G, Vural G, Dilbaz B, Akin A, Taskin L, et al. Comparison of efficacy and complications of IUD insertion in immediate postplacental/early postpartum period with interval period: 1 year follow‐up. Contraception 2006;74(5):376‐81. - PubMed
Lara Ricalde 2006 {published data only}
-
- Lara Ricalde R, Menocal Tobías G, Ramos Pérez C, Velázquez Ramírez N. Random comparative study between intrauterine device Multiload Cu375 and TCu 380a inserted in the postpartum period [Estudio comparativo al azar entre los dispositivos intrauterinos Multiload Cu375 y TCu 380A, colocados durante el posparto]. Ginecología y Obstetricia de México 2006;74(6):306‐11. - PubMed
Letti Müller 2005 {published data only}
-
- Letti Müller AL, Lopez Ramos JG, Martins‐Costa SH, Palma Dias RS, Valério EG, Hammes LS, et al. Transvaginal ultrasonographic assessment of the expulsion rate of intrauterine devices in the immediate postpartum period: a pilot study. Contraception 2005;72(3):192‐5. - PubMed
Shikary 1987 {published data only}
-
- Shikary ZK, Betrabet SS, Patel ZM, Patel S, Joshi JV, Toddywala VS, et al. Transfer of levonorgestrel (LNG) administered through different drug delivery systems from the maternal circulation into the newborn infant's circulation via breast milk. Contraception 1987;35(5):477‐86. - PubMed
Stauble 2014 {published data only}
-
- Mary E. Stauble, MD. Safety and Expulsion of Delayed Versus Immediate Postpartum Intrauterine Device Placement (IUD). http://clinicaltrials.gov/ct2/show/NCT01598662 (accessed 19 August 2014).
Stuart 2015 {published data only}
-
- Stuart G. Postpartum levonorgestrel‐releasing intrauterine system and breastfeeding (PPIUD1). http://clinicaltrials.gov/show/NCT01555931 (accessed 13 August 2014).
-
- Stuart GS, Lesko C, Stuebe A, Bryant A. Breastfeeding and postpartum insertion of the levonorgestrel intrauterine system: a randomized trial. Obstetrics and Gynecology 2014;123(Suppl 1):15S. [NCT01555931]
-
- Stuart GS, Lesko CR, Stuebe AM, Bryant AG, Levi EE, Danvers AI. A randomized trial of levonorgestrel intrauterine system insertion 6 to 48 hours compared to 6 weeks after vaginal delivery; lessons learned. Contraception 2014;91(4):284‐8. - PubMed
Tatum 1996 {published data only}
-
- Tatum HJ, Beltran RS, Ramos R, Kets H, Sivin I, Schmidt FH. Immediate postplacental insertion of GYNE‐T 380 and GYNE‐T 380 Postpartum intrauterine contraceptive devices: Randomized study. American Journal of Obstetrics and Gynecology 1996;175(5):1231‐5. - PubMed
Thiery 1983 {published data only}
-
- Thiery M, Laufe L, Parewijck W, Pas H, Kets H, Deron R, et al. Immediate postplacental IUD insertion: a randomized trial of sutured (Lippes Loop and TCu 220C) and non‐sutured (TCu 220C) models. Contraception 1983;28(4):299‐313. - PubMed
Turok 2014 {published data only}
-
- Turok D. BLIS ‐ Breastfeeding Levonorgestrel IUD Study. http://clinicaltrials.gov/show/NCT01990703 (accessed 13 August 2014). [NCT01990703]
References to studies awaiting assessment
Levi 2014 {published data only}
-
- Levi E. Immediate Postplacental Insertion of IUDs at Time of Cesarean Delivery: A Randomized Clinical Trial. http://clinicaltrials.gov/ct2/show/NCT01539759 (accessed 03 September 2014).
References to ongoing studies
Dias 2014 {published data only}
-
- Dias T. Randomised controlled trial to assess the retention of immediate post‐delivery insertion of an intrauterine contraceptive device versus interval intrauterine contraceptive device insertion. http://trials.slctr.lk/trials/231 (accessed 12 January 2015).
Harper 2014 {published data only (unpublished sought but not used)}
-
- Harper LM. Mirena Intrauterine System Timing of Insertion: A Randomized Controlled Trial (MISTIC). http://clinicaltrials.gov/ct2/show/NCT01272960 (accessed 19 August 2014).
Teal 2014b {published data only}
-
- Teal S. Comparison of the Levonorgestrel IUD and the Copper IUD Placed in the Immediate Postplacental Period: A Randomized Controlled Trial. http://clinicaltrials.gov/show/NCT02067663 (accessed 19 August 2014).
Additional references
Aiken 2014
Alam 2014
-
- Alam K, Snover A, Sultana N. Safety and feasibility of intrauterine device insertion following post‐placental delivery. Rawal Medical Journal 2014;39(2):186‐9.
Allen 2009
-
- Allen RH, Goldberg AB, Grimes DA. Expanding access to intrauterine contraception. American Journal of Obstetrics and Gynecology 2009;201(5):456.e1‐5. - PubMed
Balshem 2011
-
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. - PubMed
Bergin 2012
-
- Bergin A, Tristan S, Terplan M, Gilliam ML, Whitaker AK. A missed opportunity for care: two‐visit IUD insertion protocols inhibit placement. Contraception 2012;86(6):694‐7. - PubMed
Bryant 2013
-
- Bryant AG, Kamanga G, Stuart GS, Haddad LB, Meguid T, Mhango C. Immediate postpartum versus 6‐week postpartum intrauterine device insertion: a feasibility study of a randomized controlled trial. African Journal of Reproductive Health 2013;17(2):72‐9. - PubMed
CDC 2011
-
- Centers for Disease Control and Prevention. Update to CDC's U.S. Medical Eligibility Criteria for Contraceptive Use, 2010: revised recommendations for the use of contraceptive methods during the postpartum period. Morbidity and Mortality Weekly Report (MMWR) 2011;60(26):878‐83. - PubMed
Celen 2011
-
- Celen Ş, Sucak A, Yildiz Y, Danişman N. Immediate postplacental insertion of an intrauterine contraceptive device during cesarean section. Contraception 2011;84(3):240‐3. - PubMed
Conde‐Agudelo 2000
Echeverry 1973
-
- Echeverry G. Family planning in the immediate postpartum period. Studies in Family Planning 1973;4(2):33‐5. - PubMed
Glazer 2011
-
- Glazer AB, Wolf A, Gorby N. Postpartum contraception: needs vs. reality. Contraception 2011;83(3):238‐41. - PubMed
Gueye 2013
-
- Gueye M, Gaye YFO, Diouf AA, Mbaye M, Niang MM, Gueye SMK, et al. [Trancesarean intra‐uterine device. Pilot study performed at Dakar teaching hospital] [Dispositif intra‐utérin mis en place en cours de césarienne. Étude pilote réalisée au centre hospitalier universitaire de Dakar]. Journal de Gynecologie Obstetrique et Biologie de la Reproduction 2013;42(6):585‐90. - PubMed
Guyatt 2011
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. John Wiley & Sons, Ltd.
Kapp 2009
-
- Kapp N, Curtis KM. Intrauterine device insertion during the postpartum period: a systematic review. Contraception 2009;80(4):327‐36. - PubMed
Levi 2012
-
- Levi E, Cantillo E, Ades V, Banks E, Murthy A. Immediate postplacental IUD insertion at cesarean delivery: a prospective cohort study. Contraception 2012;86(2):102‐5. - PubMed
Lopez 2015
Mishra 2014
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schulz 2010
Shukla 2012
Sonalkar 2014
-
- Sonalkar S, Kapp N. Intrauterine device insertion in the postpartum period: a systematic review. European Journal of Contraception and Reproductive Health Care 2014;14 Nov([Epub ahead of print]):1‐15. - PubMed
Stuart 2012
Teal 2014a
-
- Teal SB. Postpartum contraception: optimizing interpregnancy intervals. Contraception 2014;89(6):487‐8. - PubMed
Thiery 1985
-
- Thiery M, Kets H, Pas H. Immediate postplacental IUD insertion: the expulsion problem. Contraception 1985;31(4):331‐49. - PubMed
Trussell 2011
UN 2011
-
- United Nations, Department of Economic and Social Affairs. World Contraceptive Use ‐ 2011. http://www.un.org/esa/population/publications/contraceptive2011/contrace... (accessed 21 Feb 2013).
WHO 2005
-
- World Health Organization. Report of a WHO Technical Consultation on Birth Spacing; 2005 Jun 13‐15; Geneva. http://www.who.int/maternal_child_adolescent/documents/birth_spacing.pdf (accessed 26 August 2014).
WHO 2009
-
- World Health Organization. Medical Eligibility Criteria for Contraceptive Use. Fourth Edition, 2009. http://www.who.int/reproductivehealth/publications/family_planning/97892.... Geneva: World Health Organization, (accessed 23 July 2014):16.
References to other published versions of this review
Grimes 2002
-
- Grimes D, Schulz K, Vliet H, Stanwood N. Immediate post‐partum insertion of intrauterine devices. Human Reproduction 2002;17:549‐54. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

