Effect of Different Adjuvants on Protection and Side-Effects Induced by Helicobacter suis Whole-Cell Lysate Vaccination
- PMID: 26115373
- PMCID: PMC4482594
- DOI: 10.1371/journal.pone.0131364
Effect of Different Adjuvants on Protection and Side-Effects Induced by Helicobacter suis Whole-Cell Lysate Vaccination
Abstract
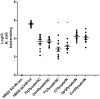
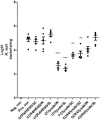
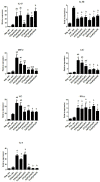
Helicobacter suis (H. suis) is a widespread porcine gastric pathogen, which is also of zoonotic importance. The first goal of this study was to investigate the efficacy of several vaccine adjuvants (CpG-DNA, Curdlan, Freund's Complete and Incomplete, Cholera toxin), administered either subcutaneously or intranasally along with H. suis whole-cell lysate, to protect against subsequent H. suis challenge in a BALB/c infection model. Subcutaneous immunization with Freund's complete (FC)/lysate and intranasal immunization with Cholera toxin (CT)/lysate were shown to be the best options for vaccination against H. suis, as determined by the amount of colonizing H. suis bacteria in the stomach, although adverse effects such as post-immunization gastritis/pseudo-pyloric metaplasia and increased mortality were observed, respectively. Therefore, we decided to test alternative strategies, including sublingual vaccine administration, to reduce the unwanted side-effects. A CCR4 antagonist that transiently inhibits the migration of regulatory T cells was also included as a new adjuvant in this second study. Results confirmed that immunization with CT (intranasally or sublingually) is among the most effective vaccination protocols, but increased mortality was still observed. In the groups immunized subcutaneously with FC/lysate and CCR4 antagonist/lysate, a significant protection was observed. Compared to the FC/lysate immunized group, gastric pseudo-pyloric metaplasia was less severe or even absent in the CCR4 antagonist/lysate immunized group. In general, an inverse correlation was observed between IFN-γ, IL-4, IL-17, KC, MIP-2 and LIX mRNA expression and H. suis colonization density, whereas lower IL-10 expression levels were observed in partially protected animals.
Conflict of interest statement
Figures





References
-
- Hellemans A, Chiers K, Maes D, De Bock M, Decostere A, Haesebrouck F, et al. (2007) Prevalence of ‘Candidatus Helicobacter suis’ in pigs of different ages. Veterinary record. 161: 189–192. - PubMed
-
- Blanchard TG, Nedrud JG. (2010) Helicobacter pylori vaccines In: Sutton P, Mitchell HM (eds.), Helicobacter pylori in the 21st century CAB International, Oxfordshire, UK, pp. 167–189.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

