Metabolomics analysis identifies novel plasma biomarkers of cystic fibrosis pulmonary exacerbation
- PMID: 26115542
- PMCID: PMC5553866
- DOI: 10.1002/ppul.23225
Metabolomics analysis identifies novel plasma biomarkers of cystic fibrosis pulmonary exacerbation
Abstract
Background: Cystic fibrosis (CF) lung disease is characterized by infection, inflammation, lung function decline, and intermittent pulmonary exacerbations. However, the link between pulmonary exacerbation and lung disease progression remains unclear. Global metabolomic profiling can provide novel mechanistic insight into a disease process in addition to putative biomarkers for future study. Our objective was to investigate how the plasma metabolomic profile changes between CF pulmonary exacerbation and a clinically well state.
Methods: Plasma samples and lung function data were collected from 25 CF patients during hospitalization for a pulmonary exacerbation and during quarterly outpatient clinic visits. In collaboration with Metabolon, Inc., the metabolomic profiles of matched pair plasma samples, one during exacerbation and one at a clinic visit, were analyzed using gas and liquid chromatography coupled with mass spectrometry. Compounds were identified by comparison to a library of standards. Mixed effects models that controlled for nutritional status and lung function were used to test for differences and principal components analysis was performed.
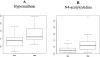
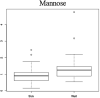
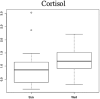
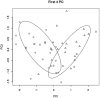
Results: Our population had a median age of 27 years (14-39) and had a median FEV1 % predicted of 65% (23-105%). 398 total metabolites were identified and after adjustment for confounders, five metabolites signifying perturbations in nucleotide (hypoxanthine), nucleoside (N4-acetylcytidine), amino acid (N-acetylmethionine), carbohydrate (mannose), and steroid (cortisol) metabolism were identified. Principal components analysis provided good separation between the two clinical phenotypes.
Conclusions: Our findings provide putative metabolite biomarkers for future study and allow for hypothesis generation about the pathophysiology of CF pulmonary exacerbation.
Keywords: infection; inflammation; lung function.
© 2015 Wiley Periodicals, Inc.
Conflict of interest statement
Conflict of interest: None.
Figures

References
-
- Welsh MJ, Ramsey BW, Accurso FJ, Cutting GR. Cystic Fibrosis Cystic Fibrosis in the Metabolic and Molecular Basis of Inherited Disease. 7th. New York: McGraw-Hill; 2001. pp. 521–588.
-
- Mott LS, Park J, Murray CP, Gangell CL, de Klerk NH, Robinson PJ, Robertson CF, Ranganathan SC, Sly PD, Stick SM, AREST CF. Progression of early structural lung disease in young children with cystic fibrosis assessed using CT. Thorax. 2012;67:509–516. - PubMed
-
- Stick SM, Brennan S, Murray C, Douglas T, von Ungern-Sternberg BS, Garratt LW, Gangell CL, De Klerk N, Linnane B, Ranganathan S, Robinson P, Robertson C, Sly PD Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF) Bronchiectasis in infants and preschool children diagnosed with cystic fibrosis after newborn screening. J Pediatr. 2009;155:623–628. - PubMed
-
- Sly PD, Brennan S, Gangell C, de Klerk N, Murray C, Mott L, Stick SM, Robinson PJ, Robertson CF, Ranganathan SC Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST-CF) Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am J Respir Crit Care Med. 2009;180:146–152. - PubMed
-
- Pillarisetti N, Williamson E, Linnane B, Skoric B, Robertson CF, Robinson P, Massie J, Hall GL, Sly P, Stick S, Ranganathan S Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF) Infection, inflammation, and lung function decline in infants with cystic fibrosis. Am J Respir Crit Care Med. 2011;184:75–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

