Tamoxifen induces a pluripotency signature in breast cancer cells and human tumors
- PMID: 26115764
- PMCID: PMC5528714
- DOI: 10.1016/j.molonc.2015.05.008
Tamoxifen induces a pluripotency signature in breast cancer cells and human tumors
Abstract
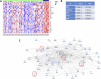
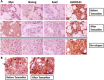
Tamoxifen is the treatment of choice in estrogen receptor alpha breast cancer patients that are eligible for adjuvant endocrine therapy. However, ∼50% of ERα-positive tumors exhibit intrinsic or rapidly acquire resistance to endocrine treatment. Unfortunately, prediction of de novo resistance to endocrine therapy and/or assessment of relapse likelihood remain difficult. While several mechanisms regulating the acquisition and the maintenance of endocrine resistance have been reported, there are several aspects of this phenomenon that need to be further elucidated. Altered metabolic fate of tamoxifen within patients and emergence of tamoxifen-resistant clones, driven by evolution of the disease phenotype during treatment, appear as the most compelling hypotheses so far. In addition, tamoxifen was reported to induce pluripotency in breast cancer cell lines, in vitro. In this context, we have performed a whole transcriptome analysis of an ERα-positive (T47D) and a triple-negative breast cancer cell line (MDA-MB-231), exposed to tamoxifen for a short time frame (hours), in order to identify how early pluripotency-related effects of tamoxifen may occur. Our ultimate goal was to identify whether the transcriptional actions of tamoxifen related to induction of pluripotency are mediated through specific ER-dependent or independent mechanisms. We report that even as early as 3 hours after the exposure of breast cancer cells to tamoxifen, a subset of ERα-dependent genes associated with developmental processes and pluripotency are induced and this is accompanied by specific phenotypic changes (expression of pluripotency-related proteins). Furthermore we report an association between the increased expression of pluripotency-related genes in ERα-positive breast cancer tissues samples and disease relapse after tamoxifen therapy. Finally we describe that in a small group of ERα-positive breast cancer patients, with disease relapse after surgery and tamoxifen treatment, ALDH1A1 (a marker of pluripotency in epithelial cancers which is absent in normal breast tissue) is increased in relapsing tumors, with a concurrent modification of its intra-cellular localization. Our data could be of value in the discrimination of patients susceptible to develop tamoxifen resistance and in the selection of optimized patient-tailored therapies.
Keywords: Breast cancer; Cell lines; Endocrine resistance; Patient data; Pluripotency; Tamoxifen; Transcriptomic analysis.
Copyright © 2015 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures



References
-
- Allahverdiyev, A. , Bagirova, M. , Nehir-Oztel, O. , Yaman, S. , Sefik-Abamor, E. , Cakir-Koc, R. , Canim-Ates, S. , Elcicek, S. , Yesilkir-Baydar, S. , 2012. Aldehyde dehydrogenase: cancer and stem cells. In Canuto R., Dehydrogenases. InTech; Rijeka, Croatia: 3–28.
-
- Ariazi, E.A. , Cunliffe, H.E. , Lewis-Wambi, J.S. , Slifker, M.J. , Willis, A.L. , Ramos, P. , Tapia, C. , Kim, H.R. , Yerrum, S. , Sharma, C.G. , Nicolas, E. , Balagurunathan, Y. , Ross, E.A. , Jordan, V.C. , 2011. Estrogen induces apoptosis in estrogen deprivation-resistant breast cancer through stress responses as identified by global gene expression across time. Proc. Natl. Acad. Sci. U S A. 108, 18879–18886. - PMC - PubMed
-
- Barnadas, A. , Estevez, L.G. , Lluch-Hernandez, A. , Rodriguez-Lescure, A. , Rodriguez-Sanchez, C. , Sanchez-Rovira, P. , 2011. An overview of letrozole in postmenopausal women with hormone-responsive breast cancer. Adv. Ther. 28, 1045–1058. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

