Size-specific follicle selection improves mouse oocyte reproductive outcomes
- PMID: 26116002
- PMCID: PMC4527888
- DOI: 10.1530/REP-15-0175
Size-specific follicle selection improves mouse oocyte reproductive outcomes
Abstract
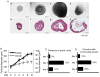
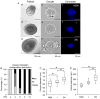
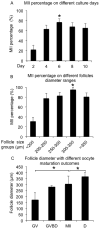
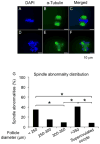
Encapsulated in vitro follicle growth (eIVFG) has great potential to provide an additional fertility preservation option for young women and girls with cancer or other reproductive health threatening diseases. Currently, follicles are cultured for a defined period of time and analyzed as a cohort. However, follicle growth is not synchronous, and culturing follicles for insufficient or excessive times can result in compromised gamete quality. Our objective is to determine whether the selection of follicles based on size, rather than absolute culture time, better predict follicle maturity and oocyte quality. Multilayer secondary mouse follicles were isolated and encapsulated in 0.25% alginate. Follicles were cultured individually either for defined time periods or up to specific follicle diameter ranges, at which point several reproductive endpoints were analyzed. The metaphase II (MII) percentage after oocyte maturation on day 6 was the highest (85%) when follicles were cultured for specific days. However, if follicles were cultured to a terminal diameter of 300-350 μm irrespective of absolute time in culture, 93% of the oocytes reached MII. More than 90% of MII oocytes matured from follicles with diameters of 300-350 μm showed normal spindle morphology and chromosome alignment, 85% of oocytes showed two pronuclei after IVF, 81% developed into the two-cell embryo stage and 38% developed to the blastocyst stage, all significantly higher than the percentages in the other follicle size groups. Our study demonstrates that size-specific follicle selection can be used as a non-invasive marker to identify high-quality oocytes and improve reproductive outcomes during eIVFG.
© 2015 Society for Reproduction and Fertility.
Conflict of interest statement
Figures
Similar articles
-
Relationship between follicular dynamics and oocyte maturation during in vitro culture as a non-invasive sign of caprine oocyte meiotic competence.Theriogenology. 2018 Feb;107:95-103. doi: 10.1016/j.theriogenology.2017.10.038. Epub 2017 Nov 4. Theriogenology. 2018. PMID: 29145066
-
Fibrin promotes development and function of macaque primary follicles during encapsulated three-dimensional culture.Hum Reprod. 2013 Aug;28(8):2187-200. doi: 10.1093/humrep/det093. Epub 2013 Apr 21. Hum Reprod. 2013. PMID: 23608357 Free PMC article.
-
A growth-maturation system that enhances the meiotic and developmental competence of porcine oocytes isolated from small follicles.Biol Reprod. 2006 Oct;75(4):547-54. doi: 10.1095/biolreprod.106.051300. Epub 2006 Jun 28. Biol Reprod. 2006. PMID: 16807383
-
Oocyte in-vitro maturation and follicle culture: current clinical achievements and future directions.Hum Reprod. 1999 Sep;14 Suppl 1:145-61. doi: 10.1093/humrep/14.suppl_1.145. Hum Reprod. 1999. PMID: 10573031 Review.
-
Ovarian follicle development in vitro and oocyte competence: advances and challenges for farm animals.Domest Anim Endocrinol. 2016 Apr;55:123-35. doi: 10.1016/j.domaniend.2015.12.006. Epub 2016 Jan 7. Domest Anim Endocrinol. 2016. PMID: 26836404 Review.
Cited by
-
Fertility preservation for adolescent and young adult cancer patients in Japan.Obstet Gynecol Sci. 2018 Jul;61(4):443-452. doi: 10.5468/ogs.2018.61.4.443. Epub 2018 Jun 19. Obstet Gynecol Sci. 2018. PMID: 30018898 Free PMC article. Review.
-
Single-cell transcriptome and cell-specific network analysis reveal the reparative effect of neurotrophin-4 in preantral follicles grown in vitro.Reprod Biol Endocrinol. 2021 Sep 4;19(1):133. doi: 10.1186/s12958-021-00818-w. Reprod Biol Endocrinol. 2021. PMID: 34481496 Free PMC article.
-
Doxorubicin Has Dose-Dependent Toxicity on Mouse Ovarian Follicle Development, Hormone Secretion, and Oocyte Maturation.Toxicol Sci. 2017 Jun 1;157(2):320-329. doi: 10.1093/toxsci/kfx047. Toxicol Sci. 2017. PMID: 28329872 Free PMC article.
-
The Steroid Metabolome in the Isolated Ovarian Follicle and Its Response to Androgen Exposure and Antagonism.Endocrinology. 2017 May 1;158(5):1474-1485. doi: 10.1210/en.2016-1851. Endocrinology. 2017. PMID: 28323936 Free PMC article.
-
In Vitro Growth of Human Follicles: Current and Future Perspectives.Int J Mol Sci. 2024 Jan 26;25(3):1510. doi: 10.3390/ijms25031510. Int J Mol Sci. 2024. PMID: 38338788 Free PMC article. Review.
References
-
- Bouniol-Baly C, Hamraoui L, Guibert J, Beaujean N, Szollosi MS, Debey P. Differential transcriptional activity associated with chromatin configuration in fully grown mouse germinal vesicle oocytes. Biology of Reproduction. 1999;60:580–587. - PubMed
-
- Carabatsos MJ, Elvin J, Matzuk MM, Albertini DF. Characterization of oocyte and follicle development in growth differentiation factor-9-deficient mice. Dev Biol. 1998;204:373–384. - PubMed
-
- De La Fuente R, Eppig JJ. Transcriptional activity of the mouse oocyte genome: companion granulosa cells modulate transcription and chromatin remodeling. Dev Biol. 2001;229:224–236. - PubMed
-
- de Moor CH, Richter JD. Translational control in vertebrate development. Int Rev Cytol. 2001;203:567–608. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

