Synthesis and preclinical evaluation of DOTAGA-conjugated PSMA ligands for functional imaging and endoradiotherapy of prostate cancer
- PMID: 26116124
- PMCID: PMC4452638
- DOI: 10.1186/s13550-014-0063-1
Synthesis and preclinical evaluation of DOTAGA-conjugated PSMA ligands for functional imaging and endoradiotherapy of prostate cancer
Abstract
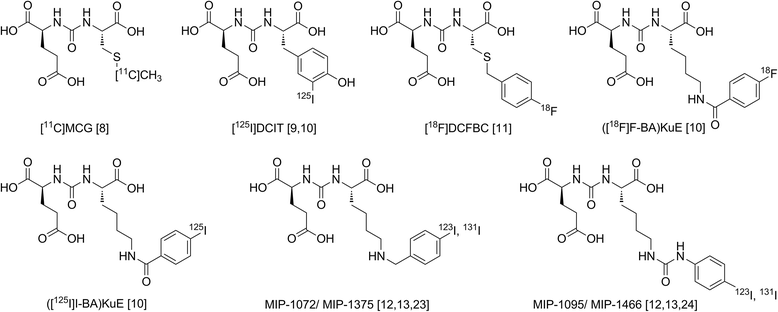
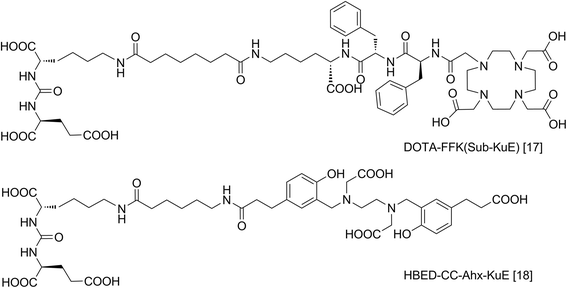
Background: Due to its high expression in prostate cancer, PSMA (prostate-specific membrane antigen) represents an ideal target for both diagnostic imaging and endoradiotherapeutic approaches. Based on a previously published highly specific PSMA ligand ([(68)Ga]DOTA-FFK(Sub-KuE)), we developed a corresponding metabolically stable 1,4,7,10-tetraazacyclododececane,1-(glutaric acid)-4,7,10-triacetic acid (DOTAGA) construct for theranostic treatment of prostate cancer.
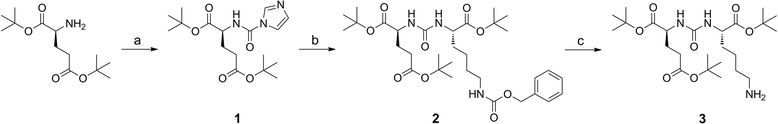
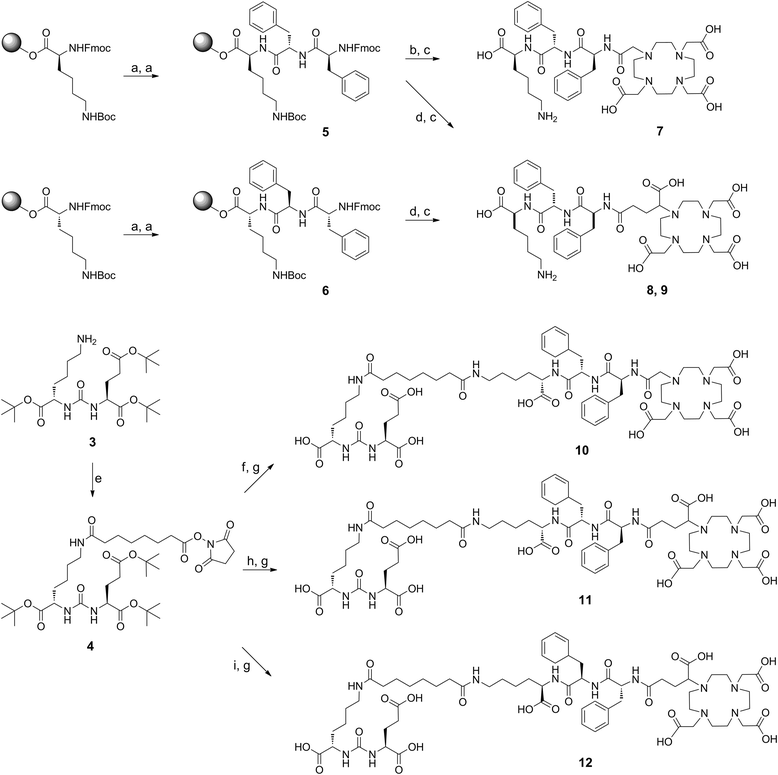
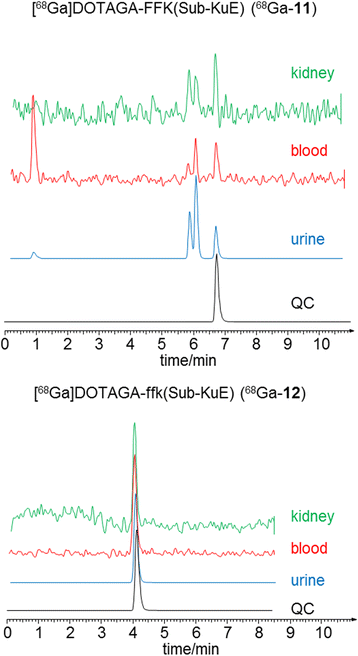
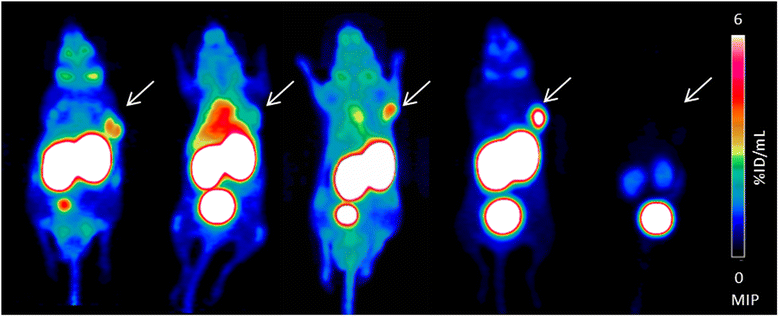
Methods: All ligands were synthesized by a combined solid phase and solution phase synthesis strategy. The affinity of the (nat)gallium and lutetium complexes to PSMA and the internalization efficiency of the radiotracers were determined on PSMA-expressing LNCaP cells. The (68)Ga- and (177)Lu-labelled ligands were further investigated for lipophilicity, binding specificity, metabolic stability, as well as biodistribution and μPET in LNCaP-tumour-bearing mice.
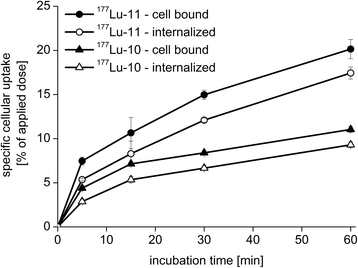
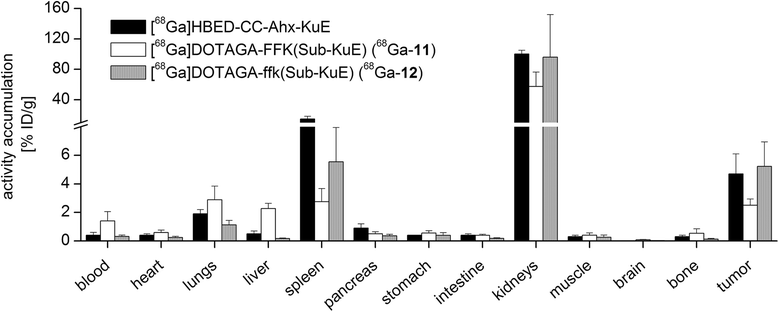
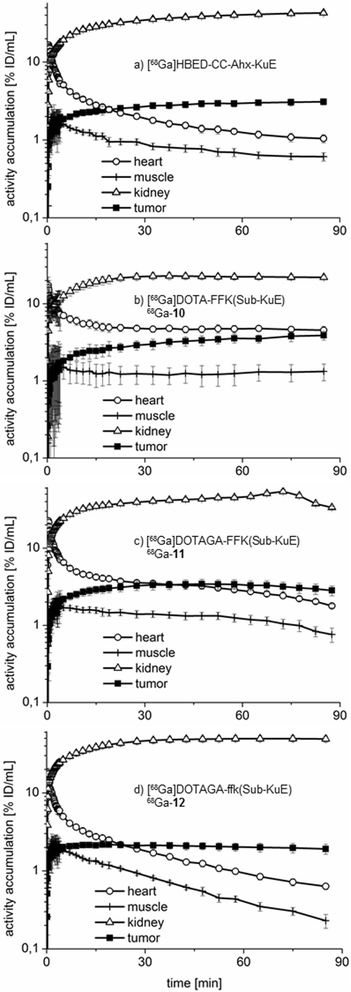
Results: Radiochemical yields for (68)Ga (3 nmol, 5.0 M NaCl/2.7 M HEPES (approximately 5/1), pH 3.5 to 4.5, 5 min, 95°C) and (177)Lu labelling (0.7 nmol, 0.1 M NH4OAc, pH 5.5, 30 min, 95°C) were almost quantitative, resulting in specific activities of 250 to 300 GBq/μmol for the (68)Ga analogues and 38 GBq/μmol for (177)Lu complexes. Due to metabolic instability of L-amino acid spacers, D-amino acids were implemented resulting in a metabolically stable DOTAGA ligand. Compared to the DOTA ligand, the DOTAGA derivatives showed higher hydrophilicity (logP = -3.6 ± 0.1 and -3.9 ± 0.1 for (68)Ga and (177)Lu, respectively) and improved affinity to PSMA resulting in an about twofold increased specific internalization of the (68)Ga- and (177)Lu-labelled DOTAGA analogue. Especially, [(68)Ga]DOTAGA-ffk(Sub-KuE) exhibits favourable pharmacokinetics, low unspecific uptake and high tumour accumulation in LNCaP-tumour-bearing mice.
Conclusions: The pair of diagnostic/therapeutic PSMA-ligands [(68)Ga/(177)Lu]DOTAGA-ffk(Sub-KuE) possess remarkable potential for the management of prostate cancer.
Figures
References
-
- Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–85. - PubMed
-
- Pinto JT, Suffoletto BP, Berzin TM, Qiao CH, Lin S, Tong WP, May F, Mukherjee B, Heston WDW. Prostate-specific membrane antigen: a novel folate hydrolase in human prostatic carcinoma cells. Clin Cancer Res. 1996;2:1445–1451. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

