Type III Secretion System Translocon Component EseB Forms Filaments on and Mediates Autoaggregation of and Biofilm Formation by Edwardsiella tarda
- PMID: 26116669
- PMCID: PMC4551241
- DOI: 10.1128/AEM.01254-15
Type III Secretion System Translocon Component EseB Forms Filaments on and Mediates Autoaggregation of and Biofilm Formation by Edwardsiella tarda
Abstract
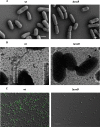
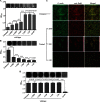
The type III secretion system (T3SS) of Edwardsiella tarda plays an important role in infection by translocating effector proteins into host cells. EseB, a component required for effector translocation, is reported to mediate autoaggregation of E. tarda. In this study, we demonstrate that EseB forms filamentous appendages on the surface of E. tarda and is required for biofilm formation by E. tarda in Dulbecco's modified Eagle's medium (DMEM). Biofilm formation by E. tarda in DMEM does not require FlhB, an essential component for assembling flagella. Dynamic analysis of EseB filament formation, autoaggregation, and biofilm formation shows that the formation of EseB filaments occurs prior to autoaggregation and biofilm formation. The addition of an EseB antibody to E. tarda cultures before bacterial autoaggregation prevents autoaggregation and biofilm formation in a dose-dependent manner, whereas the addition of the EseB antibody to E. tarda cultures in which biofilm is already formed does not destroy the biofilm. Therefore, EseB filament-mediated bacterial cell-cell interaction is a prerequisite for autoaggregation and biofilm formation.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- Koido S, Ohkusa T, Kato K, Shimamoto N, Takakura K, Odahara S, Tsukinaga S, Mitobe J, Yukawa T, Kajihara M, Uchiyama K, Arakawa H, Tajiri H. 2014. Edwardsiella tarda superinfection in relapse of ulcerative colitis. J Clin Gastroenterol 48:191–193. doi:10.1097/01.mcg.0000437809.46982.df. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

