Liposomal tetrahydrobiopterin preserves eNOS coupling in the post-ischemic heart conferring in vivo cardioprotection
- PMID: 26116866
- PMCID: PMC4558339
- DOI: 10.1016/j.yjmcc.2015.06.015
Liposomal tetrahydrobiopterin preserves eNOS coupling in the post-ischemic heart conferring in vivo cardioprotection
Abstract
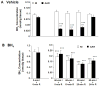
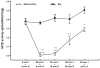
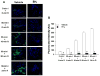
Tetrahydrobiopterin (BH4) is an essential cofactor of nitric oxide synthase (NOS), and reduced BH4 availability leads to endothelial NOS (eNOS) uncoupling and increased reactive oxygen species (ROS) generation. Questions remain regarding the functional state of eNOS and role of BH4 availability in the process of in vivo myocardial ischemia-reperfusion (I/R) injury. Rats were subjected to 60min of in vivo left coronary artery occlusion and varying periods of reperfusion with or without pre-ischemic liposomal BH4 supplementation (1mg/kg, iv). Myocardial infarction was correlated with cardiac BH4 content, eNOS protein level, NOS enzyme activity, and ROS generation. In the vehicle group, 60-min ischemia drastically reduced myocardial BH4 content in the area at risk (AAR) compared to non-ischemic (NI) area and the level remained lower during early reperfusion followed by recovery after 24-h reperfusion. Total eNOS, activated eNOS protein level (eNOS Ser1177 phosphorylation) and NOS activity were also significantly reduced during ischemia and/or early reperfusion, but recovered after 24-h reperfusion. With liposomal BH4 treatment, BH4 levels were identical in the AAR and NI area during ischemia and/or early reperfusion, and were significantly higher than with vehicle. BH4 pre-treatment preserved eNOS Ser1177 phosphorylation and NOS activity in the AAR, and significantly reduced myocardial ROS generation and infarction compared to vehicle. These findings provide direct evidence that in vivo I/R induces eNOS dysfunction secondary to BH4 depletion, and that pre-ischemic liposomal BH4 administration preserves eNOS function conferring cardioprotection with reduced oxidative stress.
Keywords: Ischemia/reperfusion; Myocardial BH(4) content; Myocardial protection; NOS activity; Oxidative stress; Tetrahydrobiopterin.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures




References
-
- Massion PB, Feron O, Dessy C, Balligand JL. Nitric oxide and cardiac function: ten years after, and continuing. Circ Res. 2003;93:388–98. - PubMed
-
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–42. - PubMed
-
- Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. The FASEB Journal. 1989;3:12. - PubMed
-
- Kwon NS, Nathan CF, Stuehr DJ. Reduced biopterin as a cofactor in the generation of nitrogen oxides by murine macrophages. J Biol Chem. 1989;264:20496–501. - PubMed
-
- Tayeh MA, Marletta MA. Macrophage oxidation of L-arginine to nitric oxide, nitrite, and nitrate. Tetrahydrobiopterin is required as a cofactor. J Biol Chem. 1989;264:19654–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

