A thioredoxin fold protein Sh3bgr regulates Enah and is necessary for proper sarcomere formation
- PMID: 26116879
- PMCID: PMC4561413
- DOI: 10.1016/j.ydbio.2015.06.005
A thioredoxin fold protein Sh3bgr regulates Enah and is necessary for proper sarcomere formation
Abstract
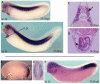
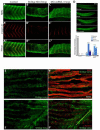
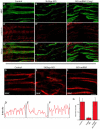
The sh3bgr (SH3 domain binding glutamate-rich) gene encodes a small protein containing a thioredoxin-like fold, SH3 binding domain, and glutamate-rich domain. Originally, it was suggested that increased expression of Sh3bgr may cause the cardiac phenotypes in Down's syndrome. However, it was recently reported that the overexpression of Sh3bgr did not cause any disease phenotypes in mice. In this study, we have discovered that Sh3bgr is critical for sarcomere formation in striated muscle tissues and also for heart development. Sh3bgr is strongly expressed in the developing somites and heart in Xenopus. Morpholino mediated-knockdown of sh3bgr caused severe malformation of heart tissue and disrupted segmentation of the somites. Further analysis revealed that Sh3bgr specifically localized to the Z-line in mature sarcomeres and that knockdown of Sh3bgr completely disrupted sarcomere formation in the somites. Moreover, overexpression of Sh3bgr resulted in abnormally discontinues thick firmaments in the somitic sarcomeres. We suggest that Sh3bgr does its function at least partly by regulating localization of Enah for the sarcomere formation. In addition, we provide the data supporting Sh3bgr is also necessary for proper heart development in part by affecting the Enah protein level.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures


References
-
- Agarkova I, Perriard JC. The M-band: an elastic web that crosslinks thick filaments in the center of the sarcomere. Trends Cell Biol. 2005;15:477–485. - PubMed
-
- Benz PM, Merkel CJ, Offner K, Abesser M, Ullrich M, Fischer T, Bayer B, Wagner H, Gambaryan S, Ursitti JA, Adham IM, Linke WA, Feller SM, Fleming I, Renne T, Frantz S, Unger A, Schuh K. Mena/VASP and alphaII-Spectrin complexes regulate cytoplasmic actin networks in cardiomyocytes and protect from conduction abnormalities and dilated cardiomyopathy. Cell Commun Signal. 2013;11:56. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

