Oncogenic miR-17/20a Forms a Positive Feed-forward Loop with the p53 Kinase DAPK3 to Promote Tumorigenesis
- PMID: 26117336
- PMCID: PMC4528155
- DOI: 10.1074/jbc.M115.661504
Oncogenic miR-17/20a Forms a Positive Feed-forward Loop with the p53 Kinase DAPK3 to Promote Tumorigenesis
Abstract
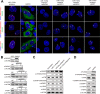
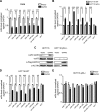
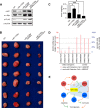
MicroRNAs (miRs) are a class of small regulatory RNAs that have been implicated in diverse biological pathways, including cancer. miR-17/20a encoded by the c13orf25 locus is among the first miRs discovered to have oncogenic functions. The E2F family members have been established as the targets for these oncomiRs, which form a negative feedback loop to control cell cycle progression. However, this pathway does not seem to be sufficient to account for elevated expression of these oncomiRs in cancer cells to promote tumorigenesis. Here we report that miR-17/20a targets a p53 activating kinase DAPK3, leading to p53-dependent transcriptional de-repression of the oncomiRs. We demonstrate that DAPK3 plays a central role in preventing miR-17/20a depletion-induced genome instability and in miR-17/20a overexpression-triggered tumor formation. This newly identified tumorigenic pathway may thus contribute to miR-17/20a amplification and tumor growth in diverse human cancers.
Keywords: DNA damage; microRNA (miRNA); oncogene; p53; tumor.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- He L., Hannon G. J. (2004) MicroRNAs: small RNAs with a big role in gene regulation. Nat. Rev. Genet. 5, 522–531 - PubMed
-
- Ota A., Tagawa H., Karnan S., Tsuzuki S., Karpas A., Kira S., Yoshida Y., Seto M. (2004) Identification and characterization of a novel gene, C13orf25, as a target for 13q31-q32 amplification in malignant lymphoma. Cancer Res. 64, 3087–3095 - PubMed
-
- Petrocca F., Vecchione A., Croce C. M. (2008) Emerging role of miR-106b-25/miR-17-92 clusters in the control of transforming growth factor beta signaling. Cancer Res. 68, 8191–8194 - PubMed
-
- Volinia S., Calin G. A., Liu C. G., Ambs S., Cimmino A., Petrocca F., Visone R., Iorio M., Roldo C., Ferracin M., Prueitt R. L., Yanaihara N., Lanza G., Scarpa A., Vecchione A., Negrini M., Harris C. C., Croce C. M. (2006) A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl. Acad. Sci. U.S.A. 103, 2257–2261 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

