Glucocerebrosidase activity in Parkinson's disease with and without GBA mutations
- PMID: 26117366
- PMCID: PMC4564023
- DOI: 10.1093/brain/awv179
Glucocerebrosidase activity in Parkinson's disease with and without GBA mutations
Abstract
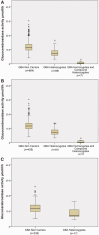
Glucocerebrosidase (GBA) mutations have been associated with Parkinson's disease in numerous studies. However, it is unknown whether the increased risk of Parkinson's disease in GBA carriers is due to a loss of glucocerebrosidase enzymatic activity. We measured glucocerebrosidase enzymatic activity in dried blood spots in patients with Parkinson's disease (n = 517) and controls (n = 252) with and without GBA mutations. Participants were recruited from Columbia University, New York, and fully sequenced for GBA mutations and genotyped for the LRRK2 G2019S mutation, the most common autosomal dominant mutation in the Ashkenazi Jewish population. Glucocerebrosidase enzymatic activity in dried blood spots was measured by a mass spectrometry-based assay and compared among participants categorized by GBA mutation status and Parkinson's disease diagnosis. Parkinson's disease patients were more likely than controls to carry the LRRK2 G2019S mutation (n = 39, 7.5% versus n = 2, 0.8%, P < 0.001) and GBA mutations or variants (seven homozygotes and compound heterozygotes and 81 heterozygotes, 17.0% versus 17 heterozygotes, 6.7%, P < 0.001). GBA homozygotes/compound heterozygotes had lower enzymatic activity than GBA heterozygotes (0.85 µmol/l/h versus 7.88 µmol/l/h, P < 0.001), and GBA heterozygotes had lower enzymatic activity than GBA and LRRK2 non-carriers (7.88 µmol/l/h versus 11.93 µmol/l/h, P < 0.001). Glucocerebrosidase activity was reduced in heterozygotes compared to non-carriers when each mutation was compared independently (N370S, P < 0.001; L444P, P < 0.001; 84GG, P = 0.003; R496H, P = 0.018) and also reduced in GBA variants associated with Parkinson's risk but not with Gaucher disease (E326K, P = 0.009; T369M, P < 0.001). When all patients with Parkinson's disease were considered, they had lower mean glucocerebrosidase enzymatic activity than controls (11.14 µmol/l/h versus 11.85 µmol/l/h, P = 0.011). Difference compared to controls persisted in patients with idiopathic Parkinson's disease (after exclusion of all GBA and LRRK2 carriers; 11.53 µmol/l/h, versus 12.11 µmol/l/h, P = 0.036) and after adjustment for age and gender (P = 0.012). Interestingly, LRRK2 G2019S carriers (n = 36), most of whom had Parkinson's disease, had higher enzymatic activity than non-carriers (13.69 µmol/l/h versus 11.93 µmol/l/h, P = 0.002). In patients with idiopathic Parkinson's, higher glucocerebrosidase enzymatic activity was associated with longer disease duration (P = 0.002) in adjusted models, suggesting a milder disease course. We conclude that lower glucocerebrosidase enzymatic activity is strongly associated with GBA mutations, and modestly with idiopathic Parkinson's disease. The association of lower glucocerebrosidase activity in both GBA mutation carriers and Parkinson's patients without GBA mutations suggests that loss of glucocerebrosidase function contributes to the pathogenesis of Parkinson's disease. High glucocerebrosidase enzymatic activity in LRRK2 G2019S carriers may reflect a distinct pathogenic mechanism. Taken together, these data suggest that glucocerebrosidase enzymatic activity could be a modifiable therapeutic target.
Keywords: LRRK2; Parkinson’s disease; genetics; glucocerebrosidase; lysosome.
© The Author (2015). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

Comment in
-
Targeting glucocerebrosidase: Reduced enzymatic activity and Parkinson's disease.Mov Disord. 2015 Oct;30(12):1620. doi: 10.1002/mds.26379. Epub 2015 Aug 11. Mov Disord. 2015. PMID: 26260953 No abstract available.
References
-
- Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R. Mutations in the glucocerebrosidase gene and Parkinson's disease in Ashkenazi Jews. N Engl J Med 2004; 351: 1972–7. doi: 351/19/1972 [pii] 10.1056/NEJMoa033277 - PubMed
-
- Balducci C, Pierguidi L, Persichetti E, Parnetti L, Sbaragli M, Tassi C, et al. Lysosomal hydrolases in cerebrospinal fluid from subjects with Parkinson's disease. Mov Disord 2007; 22: 1481–4. doi: 10.1002/mds.21399 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

