High-definition fiber tractography for the evaluation of perilesional white matter tracts in high-grade glioma surgery
- PMID: 26117712
- PMCID: PMC4588761
- DOI: 10.1093/neuonc/nov113
High-definition fiber tractography for the evaluation of perilesional white matter tracts in high-grade glioma surgery
Abstract
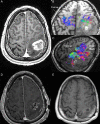
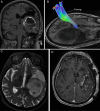
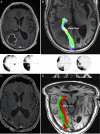
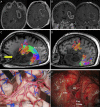
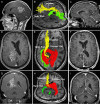
Conventional white matter (WM) imaging approaches, such as diffusion tensor imaging (DTI), have been used to preoperatively identify the location of affected WM tracts in patients with intracranial tumors in order to maximize the extent of resection and potentially reduce postoperative morbidity. DTI, however, has limitations that include its inability to resolve multiple crossing fibers and its susceptibility to partial volume effects. Therefore, recent focus has shifted to more advanced WM imaging techniques such as high-definition fiber tractography (HDFT). In this paper, we illustrate the application of HDFT, which in our preliminary experience has enabled accurate depiction of perilesional tracts in a 3-dimensional manner in multiple anatomical compartments including edematous zones around high-grade gliomas. This has facilitated accurate surgical planning. This is illustrated by using case examples of patients with glioblastoma multiforme. We also discuss future directions in the role of these techniques in surgery for gliomas.
Keywords: Diffusion tensor imaging; edema; glioblastoma multiforme; intracranial mass lesions.
© The Author(s) 2015. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
Optic radiations evaluation in patients affected by high-grade gliomas: a side-by-side constrained spherical deconvolution and diffusion tensor imaging study.Neuroradiology. 2016 Nov;58(11):1067-1075. doi: 10.1007/s00234-016-1732-8. Epub 2016 Aug 11. Neuroradiology. 2016. PMID: 27516100
-
High-definition fiber tractography of the human brain: neuroanatomical validation and neurosurgical applications.Neurosurgery. 2012 Aug;71(2):430-53. doi: 10.1227/NEU.0b013e3182592faa. Neurosurgery. 2012. PMID: 22513841
-
[Application of diffusion tensor imaging in preoperation and postoperation patients of glioma with 3.0 Tesla MRI].Zhonghua Yi Xue Za Zhi. 2009 May 19;89(19):1300-4. Zhonghua Yi Xue Za Zhi. 2009. PMID: 19615179 Chinese.
-
Tractography and the connectome in neurosurgical treatment of gliomas: the premise, the progress, and the potential.Neurosurg Focus. 2020 Feb 1;48(2):E6. doi: 10.3171/2019.11.FOCUS19785. Neurosurg Focus. 2020. PMID: 32006950 Free PMC article. Review.
-
High-Definition Fiber Tractography in Evaluation and Surgical Planning of Thalamopeduncular Pilocytic Astrocytomas in Pediatric Population: Case Series and Review of Literature.World Neurosurg. 2017 Feb;98:463-469. doi: 10.1016/j.wneu.2016.11.061. Epub 2016 Nov 22. World Neurosurg. 2017. PMID: 27888085 Review.
Cited by
-
Inferior fronto-occipital fascicle displacement in temporoinsular gliomas using diffusion tensor imaging.J Neuroimaging. 2022 Jul;32(4):638-646. doi: 10.1111/jon.12992. Epub 2022 Mar 30. J Neuroimaging. 2022. PMID: 35352437 Free PMC article.
-
A novel radiological classification system for cerebral gliomas: The Brain-Grid.PLoS One. 2019 Jan 24;14(1):e0211243. doi: 10.1371/journal.pone.0211243. eCollection 2019. PLoS One. 2019. PMID: 30677090 Free PMC article.
-
White matter tracts contribute selectively to cognitive functioning in patients with glioma.Front Oncol. 2023 Oct 20;13:1221753. doi: 10.3389/fonc.2023.1221753. eCollection 2023. Front Oncol. 2023. PMID: 37927476 Free PMC article.
-
Association between tumor architecture derived from generalized Q-space MRI and survival in glioblastoma.Oncotarget. 2017 Jun 27;8(26):41815-41826. doi: 10.18632/oncotarget.16296. Oncotarget. 2017. PMID: 28404971 Free PMC article.
-
Tumor-Associated Tractography Derived from High-Angular-Resolution Q-Space MRI May Predict Patterns of Cellular Invasion in Glioblastoma.Cancers (Basel). 2024 Oct 30;16(21):3669. doi: 10.3390/cancers16213669. Cancers (Basel). 2024. PMID: 39518107 Free PMC article.
References
-
- Le Bihan D, Breton E, Lallemand D, et al. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161(2):401–407. - PubMed
-
- Lyng H, Haraldseth O, Rofstad EK. Measurement of cell density and necrotic fraction in human melanoma xenografts by diffusion weighted magnetic resonance imaging. Magn Reson Med. 2000;43(6):828–836. - PubMed
-
- Sugahara T, Korogi Y, Kochi M, et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging. 1999;9(1):53–60. - PubMed
-
- Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103(3):247–254. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

