Decarbonylative organoboron cross-coupling of esters by nickel catalysis
- PMID: 26118733
- PMCID: PMC4491840
- DOI: 10.1038/ncomms8508
Decarbonylative organoboron cross-coupling of esters by nickel catalysis
Abstract
The Suzuki-Miyaura cross-coupling is a metal-catalysed reaction in which boron-based nucleophiles and halide-based electrophiles are reacted to form a single molecule. This is one of the most reliable tools in synthetic chemistry, and is extensively used in the synthesis of pharmaceuticals, agrochemicals and organic materials. Herein, we report a significant advance in the choice of electrophilic coupling partner in this reaction. With a user-friendly and inexpensive nickel catalyst, a range of phenyl esters of aromatic, heteroaromatic and aliphatic carboxylic acids react with boronic acids in a decarbonylative manner. Overall, phenyl ester moieties function as leaving groups. Theoretical calculations uncovered key mechanistic features of this unusual decarbonylative coupling. Since extraordinary numbers of ester-containing molecules are available both commercially and synthetically, this new 'ester' cross-coupling should find significant use in synthetic chemistry as an alternative to the standard halide-based Suzuki-Miyaura coupling.
Figures
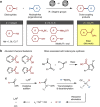


References
-
- Cross-Coupling Reactions: A Practical Guide. ed. Miyaura N. vol. 219, Springer (2002).
-
- Miyaura N. & Suzuki A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 95, 2457–2483 (1995).
-
- Lee J. C. H. & Hall D. G. Metal-Catalyzed Cross-Coupling Reactions and More eds de Meijere A., Bräse S., Oestreich M. 65–132Wiley-VCH (2014).
-
- Roglans A., Pla-Quintana A. & Moreno-Mañas M. Diazonium salts as substrates in palladium-catalyzed cross-coupling reactions. Chem. Rev. 106, 4622–4643 (2006). - PubMed
-
- Blakey S. B. & MacMillan D. W. C. The first Suzuki cross-couplings of aryltrimethylammonium salts. J. Am. Chem. Soc. 125, 6046–6047 (2003). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

