Monocyte and macrophage plasticity in tissue repair and regeneration
- PMID: 26118749
- PMCID: PMC4607753
- DOI: 10.1016/j.ajpath.2015.06.001
Monocyte and macrophage plasticity in tissue repair and regeneration
Abstract
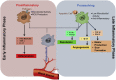
Heterogeneity and high versatility are the characteristic features of the cells of monocyte-macrophage lineage. The mononuclear phagocyte system, derived from the bone marrow progenitor cells, is primarily composed of monocytes, macrophages, and dendritic cells. In regenerative tissues, a central role of monocyte-derived macrophages and paracrine factors secreted by these cells is indisputable. Macrophages are highly plastic cells. On the basis of environmental cues and molecular mediators, these cells differentiate to proinflammatory type I macrophage (M1) or anti-inflammatory or proreparative type II macrophage (M2) phenotypes and transdifferentiate into other cell types. Given a central role in tissue repair and regeneration, the review focuses on the heterogeneity of monocytes and macrophages with current known mechanisms of differentiation and plasticity, including microenvironmental cues and molecular mediators, such as noncoding RNAs.
Copyright © 2015. Published by Elsevier Inc.
Figures


References
-
- Strauss O., Rod Dunbar P., Bartlett A., Phillips A. The immunophenotype of the antigen presenting cells of the mononuclear phagocyte system in the normal human liver: a systematic review. J Hepatol. 2015;62:458–468. - PubMed
-
- Hume D.A., Ross I.L., Himes S.R., Sasmono R.T., Wells C.A., Ravasi T. The mononuclear phagocyte system revisited. J Leukoc Biol. 2002;72:621–627. - PubMed
-
- Taylor P.R., Gordon S. Monocyte heterogeneity and innate immunity. Immunity. 2003;19:2–4. - PubMed
-
- Hume D.A. Differentiation and heterogeneity in the mononuclear phagocyte system. Mucosal Immunol. 2008;1:432–441. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

