Silencing Nociceptor Neurons Reduces Allergic Airway Inflammation
- PMID: 26119026
- PMCID: PMC4506220
- DOI: 10.1016/j.neuron.2015.06.007
Silencing Nociceptor Neurons Reduces Allergic Airway Inflammation
Abstract
Lung nociceptors initiate cough and bronchoconstriction. To elucidate if these fibers also contribute to allergic airway inflammation, we stimulated lung nociceptors with capsaicin and observed increased neuropeptide release and immune cell infiltration. In contrast, ablating Nav1.8(+) sensory neurons or silencing them with QX-314, a charged sodium channel inhibitor that enters via large-pore ion channels to specifically block nociceptors, substantially reduced ovalbumin- or house-dust-mite-induced airway inflammation and bronchial hyperresponsiveness. We also discovered that IL-5, a cytokine produced by activated immune cells, acts directly on nociceptors to induce the release of vasoactive intestinal peptide (VIP). VIP then stimulates CD4(+) and resident innate lymphoid type 2 cells, creating an inflammatory signaling loop that promotes allergic inflammation. Our results indicate that nociceptors amplify pathological adaptive immune responses and that silencing these neurons with QX-314 interrupts this neuro-immune interplay, revealing a potential new therapeutic strategy for asthma.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures
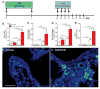




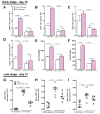
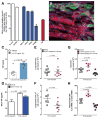

Comment in
-
Neuroimmunology: Adding insult to allergy.Nat Rev Neurosci. 2015 Aug;16(8):444. doi: 10.1038/nrn3997. Epub 2015 Jul 15. Nat Rev Neurosci. 2015. PMID: 26174707 No abstract available.
References
-
- Akinbami L. The state of childhood asthma, United States, 1980–2005. Adv Data. 2006:1–24. - PubMed
-
- Ansel JC, Brown JR, Payan DG, Brown MA. Substance P selectively activates TNF-alpha gene expression in murine mast cells. J Immunol. 1993;150:4478–4485. - PubMed
-
- Barnes PJ. Neuroeffector mechanisms: the interface between inflammation and neuronal responses. J Allergy Clin Immunol. 1996;98:S73–81. discussion S81–73. - PubMed
-
- Barnes PJ. New drugs for asthma. Nat Rev Drug Discov. 2004;3:831–844. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 HL007633/HL/NHLBI NIH HHS/United States
- R37 NS039518/NS/NINDS NIH HHS/United States
- R37NS039518/NS/NINDS NIH HHS/United States
- P01-GM095467/GM/NIGMS NIH HHS/United States
- R01 AI068084/AI/NIAID NIH HHS/United States
- P01 GM095467/GM/NIGMS NIH HHS/United States
- 5T32HL007633/HL/NHLBI NIH HHS/United States
- F32 NS076297-01/NS/NINDS NIH HHS/United States
- P01 NS072040/NS/NINDS NIH HHS/United States
- P30 HD018655/HD/NICHD NIH HHS/United States
- R01-HL122531/HL/NHLBI NIH HHS/United States
- AI068084/AI/NIAID NIH HHS/United States
- R01 HL122531/HL/NHLBI NIH HHS/United States
- K22 AI114810/AI/NIAID NIH HHS/United States
- F32 NS076297/NS/NINDS NIH HHS/United States
- P01NS072040/NS/NINDS NIH HHS/United States
- R56 AI068084/AI/NIAID NIH HHS/United States
- P30-HD18655/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

