Insights into the Mechanisms Involved in the Expression and Regulation of Extracellular Matrix Proteins in Diabetic Nephropathy
- PMID: 26119175
- PMCID: PMC4863711
- DOI: 10.2174/0929867322666150625095407
Insights into the Mechanisms Involved in the Expression and Regulation of Extracellular Matrix Proteins in Diabetic Nephropathy
Abstract
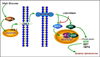
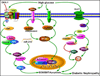
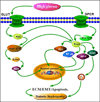
Diabetic Nephropathy (DN) is believed to be a major microvascular complication of diabetes. The hallmark of DN includes deposition of Extracellular Matrix (ECM) proteins, such as, collagen, laminin and fibronectin in the mesangium and renal tubulo-interstitium of the glomerulus and basement membranes. Such an increased expression of ECM leads to glomerular and tubular basement membranes thickening and increase of mesangial matrix, ultimately resulting in glomerulosclerosis and tubulointerstitial fibrosis. The characteristic morphologic glomerular mesangial lesion has been described as Kimmelstiel-Wilson nodule, and the process at times is referred to as diabetic nodular glomerulosclerosis. Thus, the accumulation of ECM proteins plays a critical role in the development of DN. The relevant mechanism(s) involved in the increased ECM expression and their regulation in the kidney in diabetic state has been extensively investigated and documented in the literature. Nevertheless, there are certain other mechanisms that may yet be conclusively defined. Recent studies demonstrated that some of the new signaling pathways or molecules including, Notch, Wnt, mTOR, TLRs and small GTPase may play a pivotal role in the modulation of ECM regulation and expression in DN. Such modulation could be operational for instance Notch through Notch1/Jagged1 signaling, Wnt by Wnt/β- catenin pathway and mTOR via PI3-K/Akt/mTOR signaling pathways. All these pathways may be critical in the modulation of ECM expression and tubulo-interstitial fibrosis. In addition, TLRs, mainly the TLR2 and TLR4, by TLR2- dependent and TGF-β-dependent conduits, may modulate ECM expression and generate a fibrogenic response. Small GTPase like Rho, Ras and Rab family by targeting relevant genes may also influence the accumulation of ECM proteins and renal fibrosis in hyperglycemic states. This review summarizes the recent information about the role and mechanisms by which these molecules and signaling pathways regulate ECM synthesis and its expression in high glucose ambience in vitro and in vivo states. The understanding of such signaling pathways and the molecules that influence expression, secretion and amassing of ECM may aid in developing strategies for the amelioration of diabetic nephropathy.
Conflict of interest statement
Figures
References
-
- Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, Chugh S, Danesh FR. Diabetic nephropathy: mechanisms of renal disease progression. Exp. Biol. Med. (Maywood) 2008;233(1):4–11. - PubMed
-
- Murphy M, Crean J, Brazil DP, Sadlier D, Martin F, Godson C. Regulation and consequences of differential gene expression in diabetic kidney disease. Biochem. Soc. Trans. 2008;36(Pt 5):941–945. - PubMed
-
- Haider DG, Peric S, Friedl A, Fuhrmann V, Wolzt M, Horl WH, Soleiman A. Kidney biopsy in patients with diabetes mellitus. Clin. Nephrol. 2011;76(3):180–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

