Stimulation of EphB2 attenuates tau phosphorylation through PI3K/Akt-mediated inactivation of glycogen synthase kinase-3β
- PMID: 26119563
- PMCID: PMC4484244
- DOI: 10.1038/srep11765
Stimulation of EphB2 attenuates tau phosphorylation through PI3K/Akt-mediated inactivation of glycogen synthase kinase-3β
Abstract
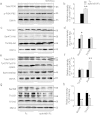
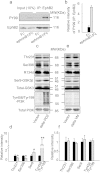
Abnormal tau hyperphosphorylation is an early pathological marker of Alzheimer's disease (AD), however, the upstream factors that regulate tau phosphorylation are not illustrated and there is no efficient strategy to arrest tau hyperphosphorylation. Here, we find that activation of endogenous EphB2 receptor by ligand stimulation (ephrinB1/Fc) or by ectopic expression of EphB2 plus the ligand stimulation induces a remarkable tau dephosphorylation at multiple AD-associated sites in SK-N-SH cells and human embryonic kidney cells that stably express human tau (HEK293-tau). In cultured hippocampal neurons and the hippocampus of human tau transgenic mice, dephosphorylation of tau proteins was also detected by stimulation of EphB2 receptor. EphB2 activation inhibits glycogen synthase kinase-3β (GSK-3β), a crucial tau kinase, and activates phosphatidylinositol-3-kinase (PI3K)/Akt both in vitro and in vivo, whereas simultaneous inhibition of PI3K or upregulation of GSK-3β abolishes the EphB2 stimulation-induced tau dephosphorylation. Finally, we confirm that ephrinB1/Fc treatment induces tyrosine phosphorylation (activation) of EphB2, while deletion of the tyrosine kinase domain (VM) of EphB2 eliminates the receptor stimulation-induced GSK-3β inhibition and tau dephosphorylation. We conclude that activation of EphB2 receptor kinase arrests tau hyperphosphorylation through PI3K-/Akt-mediated GSK-3β inhibition. Our data provide a novel membranous target to antagonize AD-like tau pathology.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

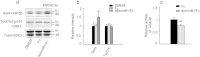


References
-
- Braak H. & Del Tredici K. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta neuropathologica 121, 171–181 (2011). - PubMed
-
- Lee V. M., Balin B. J., Otvos L. Jr. & Trojanowski J. Q. A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science 251, 675–678 (1991). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

