Duplex-selective ruthenium-based DNA intercalators
- PMID: 26119581
- PMCID: PMC4808573
- DOI: 10.1002/chem.201502095
Duplex-selective ruthenium-based DNA intercalators
Abstract
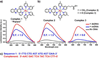
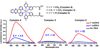
We report the design and synthesis of small molecules that exhibit enhanced luminescence in the presence of duplex rather than single-stranded DNA. The local environment presented by a well-known [Ru(dipyrido[3,2-a:2',3'-c]phenazine)L2 ](2+) -based DNA intercalator was modified by functionalizing the bipyridine ligands with esters and carboxylic acids. By systematically varying the number and charge of the pendant groups, it was determined that decreasing the electrostatic interaction between the intercalator and the anionic DNA backbone reduced single-strand interactions and translated to better duplex specificity. In studying this class of complexes, a single Ru(II) complex emerged that selectively luminesces in the presence of duplex DNA with little to no background from interacting with single-stranded DNA. This complex shows promise as a new dye capable of selectively staining double- versus single-stranded DNA in gel electrophoresis, which cannot be done with conventional SYBR dyes.
Keywords: DNA; bioinorganic chemistry; gels; luminescence; ruthenium.
© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
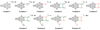


References
-
- Southern EM. J. Mol. Biol. 1975;98:503–517. - PubMed
-
- Friedman AE, Chambron JC, Sauvage JP, Turro NJ, Barton JK. J. Am. Chem. Soc. 1990;112:4960–4962.
- Amouyal E, Homsi A, Chambron J-C, Sauvage J-P. J. Chem. Soc., Dalton Trans. 1990:1841–1845.
- Hartshorn RM, Barton JK. J. Am. Chem. Soc. 1992;114:5919–5925.
- Keene FR, Smith JA, Collins JG. Coord. Chem. Rev. 2009;253:2021–2035.
-
- Della Ciana L, Hamachi I, Meyer TJ. J. Org. Chem. 1989;54:1731–1735.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

