Hexaarylbiimidazoles as visible light thiol-ene photoinitiators
- PMID: 26119702
- PMCID: PMC4605678
- DOI: 10.1016/j.dental.2015.06.005
Hexaarylbiimidazoles as visible light thiol-ene photoinitiators
Abstract
Objectives: The aim of this study is to determine if hexaarylbiimidazoles (HABIs) are efficient, visible light-active photoinitiators for thiol-ene systems. We hypothesize that, owing to the reactivity of lophyl radicals with thiols and the necessarily high concentration of thiol in thiol-ene formulations, HABIs will effectively initiate thiol-ene polymerization upon visible light irradiation.
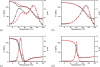
Methods: UV-vis absorption spectra of photoinitiator solutions were obtained using UV-vis spectroscopy, while EPR spectroscopy was used to confirm radical species generation upon HABI photolysis. Functional group conversions during photopolymerization were monitored using FTIR spectroscopy, and thermomechanical properties were determined using dynamic mechanical analysis.
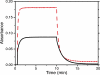
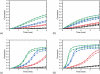
Results: The HABI derivatives investigated exhibit less absorptivity than camphorquinone at 469nm; however, they afford increased sensitivity at this wavelength when compared with bis(2,4,6-trimethylbenzoyl)-phenylphosphineoxide. Photolysis of the investigated HABIs affords lophyl radicals. Affixing hydroxyhexyl functional groups to the HABI core significantly improved solubility. Thiol-ene resins formulated with HABI photoinitiators polymerized rapidly upon irradiation with 469nm. The glass transition temperatures of the thiol-ene resin formulated with a bis(hydroxyhexyl)-functionalized HABI and photopolymerized at room and body temperature were 49.5±0.5°C and 52.2±0.1°C, respectively.
Significance: Although thiol-enes show promise as continuous phases for composite dental restorative materials, they show poor reactivity with the conventional camphorquinone/tertiary amine photoinitiation system. Conversely, despite their relatively low visible light absorptivity, HABI photoinitiators afford rapid thiol-ene photopolymerization rates. Moreover, minor structural modifications suggest pathways for improved HABI solubility and visible light absorption.
Keywords: Camphorquinone; HABI; Photoinitiator; Thiol–ene.
Copyright © 2015 Academy of Dental Materials. Published by Elsevier Ltd. All rights reserved.
Figures








References
-
- Peutzfeldt A. Resin composites in dentistry: The monomer systems. Eur J Oral Sci. 1997;105:97–116. - PubMed
-
- Ferracane JL. Resin composite-State of the art. Dental Materials. 2011;27:29–38. - PubMed
-
- Rueggeberg FA, Margeson DH. The effect of oxygen inhibition on an unfilled/filled composite system. Journal of dental research. 1990;69:1652–8. - PubMed
-
- Gauthier MA, Stangel I, Ellis TH, Zhu XX. Oxygen inhibition in dental resins. Journal of Dental Research. 2005;84:725–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

