The Mitochondrial Calcium Uniporter Matches Energetic Supply with Cardiac Workload during Stress and Modulates Permeability Transition
- PMID: 26119731
- PMCID: PMC4517182
- DOI: 10.1016/j.celrep.2015.06.017
The Mitochondrial Calcium Uniporter Matches Energetic Supply with Cardiac Workload during Stress and Modulates Permeability Transition
Abstract
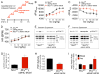
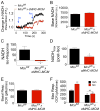
Cardiac contractility is mediated by a variable flux in intracellular calcium (Ca(2+)), thought to be integrated into mitochondria via the mitochondrial calcium uniporter (MCU) channel to match energetic demand. Here, we examine a conditional, cardiomyocyte-specific, mutant mouse lacking Mcu, the pore-forming subunit of the MCU channel, in adulthood. Mcu(-/-) mice display no overt baseline phenotype and are protected against mCa(2+) overload in an in vivo myocardial ischemia-reperfusion injury model by preventing the activation of the mitochondrial permeability transition pore, decreasing infarct size, and preserving cardiac function. In addition, we find that Mcu(-/-) mice lack contractile responsiveness to acute β-adrenergic receptor stimulation and in parallel are unable to activate mitochondrial dehydrogenases and display reduced bioenergetic reserve capacity. These results support the hypothesis that MCU may be dispensable for homeostatic cardiac function but required to modulate Ca(2+)-dependent metabolism during acute stress.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



References
-
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. - PubMed
-
- Bers DM. Calcium cycling and signaling in cardiac myocytes. Annual review of physiology. 2008;70:23–49. - PubMed
-
- Beutner G, Sharma VK, Giovannucci DR, Yule DI, Sheu SS. Identification of a ryanodine receptor in rat heart mitochondria. The Journal of biological chemistry. 2001;276:21482–21488. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

