Characterization of cells from patient-derived fibrovascular membranes in proliferative diabetic retinopathy
- PMID: 26120272
- PMCID: PMC4462955
Characterization of cells from patient-derived fibrovascular membranes in proliferative diabetic retinopathy
Abstract
Purpose: Epiretinal fibrovascular membranes (FVMs) are a hallmark of proliferative diabetic retinopathy (PDR). Surgical removal of FVMs is often indicated to treat tractional retinal detachment. This potentially informative pathological tissue is usually disposed of after surgery without further examination. We developed a method for isolating and characterizing cells derived from FVMs and correlated their expression of specific markers in culture with that in tissue.
Methods: FVMs were obtained from 11 patients with PDR during diabetic vitrectomy surgery and were analyzed with electron microscopy (EM), comparative genomic hybridization (CGH), immunohistochemistry, and/or digested with collagenase II for cell isolation and culture. Antibody arrays and enzyme-linked immunosorbent assay (ELISA) were used to profile secreted angiogenesis-related proteins in cell culture supernatants.
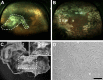
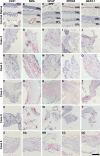
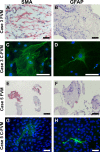
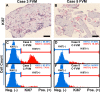
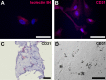
Results: EM analysis of the FVMs showed abnormal vessels composed of endothelial cells with large nuclei and plasma membrane infoldings, loosely attached perivascular cells, and stromal cells. The cellular constituents of the FVMs lacked major chromosomal aberrations as shown with CGH. Cells derived from FVMs (C-FVMs) could be isolated and maintained in culture. The C-FVMs retained the expression of markers of cell identity in primary culture, which define specific cell populations including CD31-positive, alpha-smooth muscle actin-positive (SMA), and glial fibrillary acidic protein-positive (GFAP) cells. In primary culture, secretion of angiopoietin-1 and thrombospondin-1 was significantly decreased in culture conditions that resemble a diabetic environment in SMA-positive C-FVMs compared to human retinal pericytes derived from a non-diabetic donor.
Conclusions: C-FVMs obtained from individuals with PDR can be isolated, cultured, and profiled in vitro and may constitute a unique resource for the discovery of cell signaling mechanisms underlying PDR that extends beyond current animal and cell culture models.
Figures

References
-
- Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–36. - PubMed
-
- Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol. 1984;102:527–32. - PubMed
-
- Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O'Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64. - PMC - PubMed
-
- Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366:1227–39. - PubMed
-
- Eliott D, Lee M, Abrams GW. Proliferative diabetic retinopathy: principles and techniques of surgery. In: Ryan SJ, editor. Retina. 4th ed: Elsevier; 2005.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

