Alzheimer's disease progression by geographical region in a clinical trial setting
- PMID: 26120369
- PMCID: PMC4481070
- DOI: 10.1186/s13195-015-0127-0
Alzheimer's disease progression by geographical region in a clinical trial setting
Abstract
Introduction: To facilitate enrollment and meet local registration requirements, sponsors have increasingly implemented multi-national Alzheimer's disease (AD) studies. Geographic regions vary on many dimensions that may affect disease progression or its measurement. To aid researchers designing and implementing Phase 3 AD trials, we assessed disease progression across geographic regions using placebo data from four large, multi-national clinical trials of investigational compounds developed to target AD pathophysiology.
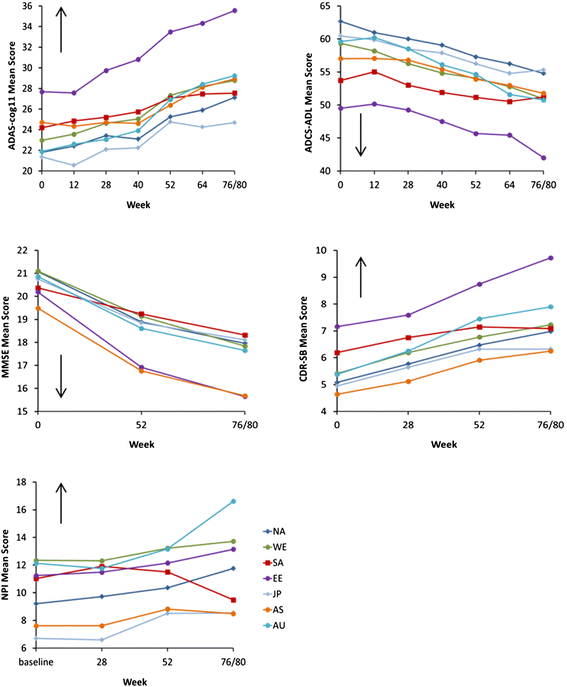
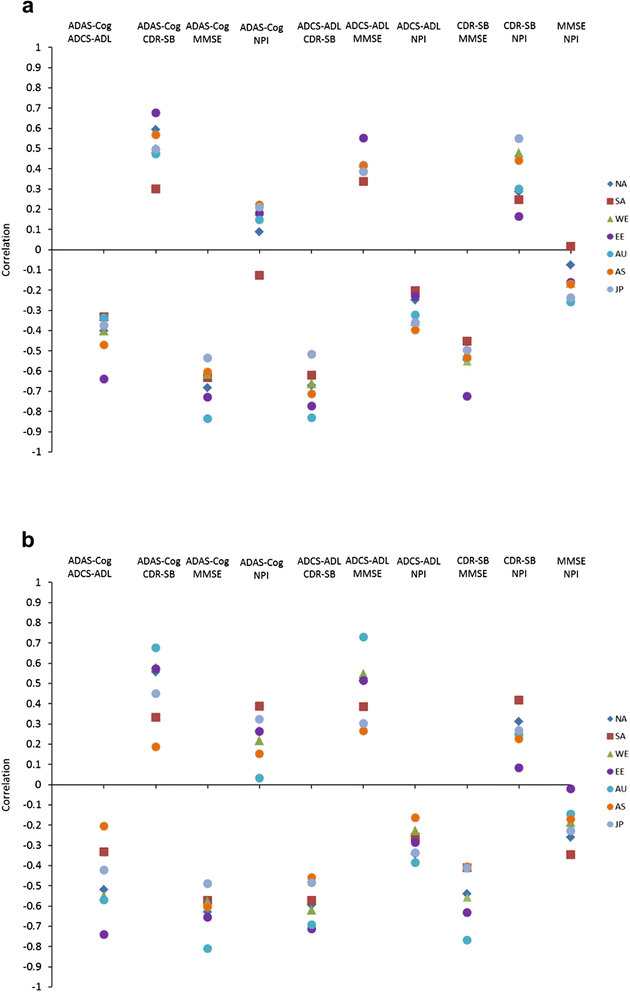
Methods: Four similarly-designed 76 to 80 week, randomized, double-blind placebo-controlled trials with nearly identical entry criteria enrolled patients aged ≥55 years with mild or moderate NINCDS/ADRDA probable AD. Descriptive analyses were performed for observed mean score and observed mean change in score from baseline at each scheduled visit. Data included in the analyses were pooled from the intent-to-treat placebo-assigned overall (mild and moderate) AD dementia populations from all four studies. Disease progression was assessed as change from baseline for each of 5 scales - the AD Assessment Scale-cognitive subscale (ADAS-cog11), the AD Cooperative Study- Activities of Daily Living Scale (ADCS-ADL), Mini-Mental State Examination (MMSE), the Clinical Dementia Rating scored by the sum of boxes method (CDR-SB), and the Neuropsychiatric Inventory (NPI).
Results: Regions were heterogeneous at baseline. At baseline, disease severity as measured by ADAS-cog11, ADCS-ADL, and CDR-SB was numerically worse for Eastern Europe/Russia compared with other regions. Of all regional populations, Eastern Europe/Russia showed the greatest cognitive and functional decline from baseline; Japan, Asia and/or S. America/Mexico showed the least cognitive and functional decline.
Conclusions: These data suggest that in multi-national clinical trials, AD progression or its measurement may differ across geographic regions; this may be in part due to heterogeneity across populations at baseline. The observed differences in AD progression between outcome measures across geographic regions may generalize to 'real-world' clinic populations, where heterogeneity is the norm.
Trial registrations: ClinicalTrials.gov NCT00594568 - IDENTITY. Registered 11 January 2008. ClinicalTrials.gov NCT00762411 - IDENTITY2. Registered 26 September 2008 ClinicalTrials.gov NCT00905372 - EXPEDITION. Registered 18 May 2009 ClinicalTrials.gov NCT00904683 - EXPEDITION2. Registered 18 May 2009.
Figures
References
-
- Degenhardt EK, Witte MM, Case MG, Yu P, Henley DB, Hochstetler HM, et al. Florbetapir F18 PET amyloid neuroimaging and baseline characteristics in patients with Alzheimer’s dementia [abstract]. Neurology. 2014;82:P3.209.
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

