Association of a Methanol Extract of Rheum undulatum L. Mediated Cell Death in AGS Cells with an Intrinsic Apoptotic Pathway
- PMID: 26120485
- PMCID: PMC4481396
- DOI: 10.3831/KPI.2015.18.012
Association of a Methanol Extract of Rheum undulatum L. Mediated Cell Death in AGS Cells with an Intrinsic Apoptotic Pathway
Abstract
Objectives: Rheum undulatum L. has traditionally been used for the treatment of many diseases in Asia. However, its anti-proliferative activity in cancer has still not been studied. In the present study, we investigated the anti-cancer effects of methanol extract of Rheum undulatum L. (MERL) on human adenocarcinoma gastric cell lines (AGS).
Methods: To investigate the anti-cancer effect of MERL on AGS cells, we treated the AGS cells with varying con¬centrations of MERL and performed 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assays. Cell cycle analyses, measurements of the mitochondrial membrane potential (MMP), caspase activity assays and Western blots were conducted to determine whether AGS cell death occurred by apoptosis.
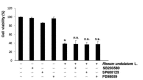
Results: Treatment with MERL significantly inhibited growth of AGS cells in a concentration dependent manner. MERL treatment in AGS cells leaded to increased accumulation of apoptotic sub G1 phase cells in a concentration dependent manner. In control cultures, 5.38% of the cells were in the sub G1 phase. In MERL treated cells, however, this percentage was significantly increased (9.95% at 70 μg/mL, 15.94% at 140 μg/mL, 26.56% at 210 μg/mL and 38.08% at 280 μg/mL). MERL treatment induced the decreased expression of pro-caspase-8 and -9 in a concentration dependent manner, whereas the expression of the active form of caspase-3 was increased. A subsequent Western blot analysis revealed increased cleaved levels of poly (ADP-ribose) polymerase (PARP) protein. Also, treatment with MERL increased the activities of caspase-3 and -9 compared with the control. MERL treatment increased the levels of the pro-apoptotic truncated Bid (tBid) and Bcl2 Antagonist X (Bax) proteins and decreased the levels of the anti-apoptotic B-cell lymphoma 2 (Bcl-2) protein, whose is the stabilization of mitochondria. However, inhibitions of p38, extracellular signal regulated kinases (ERKs) and C-Jun N-terminal kinases (JNK) by MERL treatment did not affect cell death.
Conclusion: These results suggest that MERL mediated cell death is associated with an intrinsic apoptotic pathway in AGS cells.
Keywords: AGS human gastric adenocarcinoma cells; Rheum undulatum L; anti-proliferative activity; apoptosis; cell death; intrinsic apoptotic pathway.
Conflict of interest statement
Figures




Similar articles
-
Apoptosis of AGS human gastric adenocarcinoma cells by methanolic extract of Dictamnus.Pharmacogn Mag. 2015 Oct;11(Suppl 2):S329-36. doi: 10.4103/0973-1296.165994. Pharmacogn Mag. 2015. PMID: 26664023 Free PMC article.
-
Induction of apoptosis and G1 phase cell cycle arrest by Aster incisus in AGS gastric adenocarcinoma cells.Int J Oncol. 2018 Nov;53(5):2300-2308. doi: 10.3892/ijo.2018.4547. Epub 2018 Aug 30. Int J Oncol. 2018. PMID: 30226597
-
Poncirin Induces Apoptosis in AGS Human Gastric Cancer Cells through Extrinsic Apoptotic Pathway by up-Regulation of Fas Ligand.Int J Mol Sci. 2015 Sep 18;16(9):22676-91. doi: 10.3390/ijms160922676. Int J Mol Sci. 2015. PMID: 26393583 Free PMC article.
-
Stilbenes contribute to the anticancer effects of Rheum undulatum L. through activation of apoptosis.Oncol Lett. 2019 Mar;17(3):2953-2959. doi: 10.3892/ol.2019.9926. Epub 2019 Jan 14. Oncol Lett. 2019. PMID: 30854073 Free PMC article.
-
Cycloartobiloxanthone Induces Human Lung Cancer Cell Apoptosis via Mitochondria-dependent Apoptotic Pathway.In Vivo. 2018 Jan-Feb;32(1):71-78. doi: 10.21873/invivo.11206. In Vivo. 2018. PMID: 29275301 Free PMC article.
Cited by
-
Varietal Differences in Juice, Pomace and Root Biochemical Characteristics of Four Rhubarb (Rheum rhabarbarum L.) Cultivars.BioTech (Basel). 2023 Jan 19;12(1):12. doi: 10.3390/biotech12010012. BioTech (Basel). 2023. PMID: 36810439 Free PMC article.
-
An Investigation of the Antigastric Cancer Effect in Tumor Microenvironment of Radix Rhei Et Rhizome: A Network Pharmacology Study.Evid Based Complement Alternat Med. 2021 Jun 24;2021:9913952. doi: 10.1155/2021/9913952. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 34257692 Free PMC article.
-
Neuroprotective Effects of Lindleyin on Hydrogen Peroxide-Induced Cell Injury and MPTP-Induced Parkinson's Disease in C57BL/6 Mice.Evid Based Complement Alternat Med. 2020 Feb 28;2020:2938432. doi: 10.1155/2020/2938432. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 32190079 Free PMC article.
-
Comparison of the Phytochemical Properties, Antioxidant Activity and Cytotoxic Effect on HepG2 Cells in Mongolian and Taiwanese Rhubarb Species.Molecules. 2021 Feb 25;26(5):1217. doi: 10.3390/molecules26051217. Molecules. 2021. PMID: 33668690 Free PMC article.
-
c9, t11- conjugated linoleic acid induces HCC cell apoptosis and correlation with PPAR-γ signaling pathway.Am J Transl Res. 2015 Dec 15;7(12):2752-63. eCollection 2015. Am J Transl Res. 2015. PMID: 26885272 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
