Different immune cells mediate mechanical pain hypersensitivity in male and female mice
- PMID: 26120961
- PMCID: PMC4772157
- DOI: 10.1038/nn.4053
Different immune cells mediate mechanical pain hypersensitivity in male and female mice
Abstract
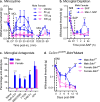
A large and rapidly increasing body of evidence indicates that microglia-to-neuron signaling is essential for chronic pain hypersensitivity. Using multiple approaches, we found that microglia are not required for mechanical pain hypersensitivity in female mice; female mice achieved similar levels of pain hypersensitivity using adaptive immune cells, likely T lymphocytes. This sexual dimorphism suggests that male mice cannot be used as proxies for females in pain research.
Conflict of interest statement
The authors have no competing interests as defined by Nature Publishing Group, or other interests that might be perceived to influence the results and/or discussion reported in this article.
Figures
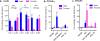
Comment in
-
Neuroimmunology: A painful difference between the sexes.Nat Rev Immunol. 2015 Aug;15(8):469. doi: 10.1038/nri3892. Epub 2015 Jul 17. Nat Rev Immunol. 2015. PMID: 26184714 No abstract available.
-
Sex, drugs and pain control.Nat Neurosci. 2015 Aug;18(8):1059-60. doi: 10.1038/nn.4057. Nat Neurosci. 2015. PMID: 26216458 Free PMC article.
References
-
- Watkins LR, Maier SF. Glia: a novel drug discovery target for clinical pain. Nat Rev Drug Discov. 2003;2:973–985. - PubMed
-
- Mogil JS, Chanda ML. The case for the inclusion of female subjects in basic science studies of pain. Pain. 2005;117:1–5. - PubMed
-
- Tsuda M, et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

