Nuclear pore complex remodeling by p75(NTR) cleavage controls TGF-β signaling and astrocyte functions
- PMID: 26120963
- PMCID: PMC4878404
- DOI: 10.1038/nn.4054
Nuclear pore complex remodeling by p75(NTR) cleavage controls TGF-β signaling and astrocyte functions
Abstract
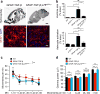
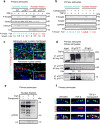
Astrocytes modulate neuronal activity and inhibit regeneration. We show that cleaved p75 neurotrophin receptor (p75(NTR)) is a component of the nuclear pore complex (NPC) required for glial scar formation and reduced gamma oscillations in mice via regulation of transforming growth factor (TGF)-β signaling. Cleaved p75(NTR) interacts with nucleoporins to promote Smad2 nucleocytoplasmic shuttling. Thus, NPC remodeling by regulated intramembrane cleavage of p75(NTR) controls astrocyte-neuronal communication in response to profibrotic factors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- 8P41GM103412-24/GM/NIGMS NIH HHS/United States
- P41 GM103412/GM/NIGMS NIH HHS/United States
- 5P41RR004050-24/RR/NCRR NIH HHS/United States
- R21 NS082976/NS/NINDS NIH HHS/United States
- T32 AI007334/AI/NIAID NIH HHS/United States
- R21NS082976/NS/NINDS NIH HHS/United States
- AG047313/AG/NIA NIH HHS/United States
- R01NS066361/NS/NINDS NIH HHS/United States
- R01 NS066361/NS/NINDS NIH HHS/United States
- P41 RR004050/RR/NCRR NIH HHS/United States
- R01 NS052189/NS/NINDS NIH HHS/United States
- R01 NS051470/NS/NINDS NIH HHS/United States
- R01 AG047313/AG/NIA NIH HHS/United States
- R01NS052189/NS/NINDS NIH HHS/United States
- R01NS051470/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

