Elevated Intracranial Pressure and Cerebral Edema following Permanent MCA Occlusion in an Ovine Model
- PMID: 26121036
- PMCID: PMC4486455
- DOI: 10.1371/journal.pone.0130512
Elevated Intracranial Pressure and Cerebral Edema following Permanent MCA Occlusion in an Ovine Model
Abstract
Introduction: Malignant middle cerebral artery (MCA) stroke has a disproportionately high mortality due to the rapid development of refractory space-occupying cerebral edema. Animal models are essential in developing successful anti-edema therapies; however to date poor clinical translation has been associated with the predominately used rodent models. As such, large animal gyrencephalic models of stroke are urgently needed. The aim of the study was to characterize the intracranial pressure (ICP) response to MCA occlusion in our recently developed ovine stroke model.
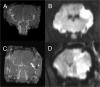
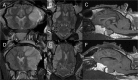
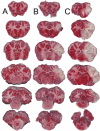
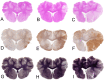
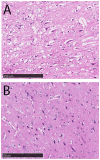
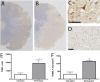
Materials and methods: 30 adult female Merino sheep (n = 8-12/gp) were randomized to sham surgery, temporary or permanent proximal MCA occlusion. ICP and brain tissue oxygen were monitored for 24 hours under general anesthesia. MRI, infarct volume with triphenyltetrazolium chloride (TTC) staining and histology were performed.
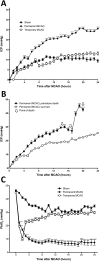
Results: No increase in ICP, radiological evidence of ischemia within the MCA territory but without space-occupying edema, and TTC infarct volumes of 7.9+/-5.1% were seen with temporary MCAO. Permanent MCAO resulted in significantly elevated ICP, accompanied by 30% mortality, radiological evidence of space-occupying cerebral edema and TTC infarct volumes of 27.4+/-6.4%.
Conclusions: Permanent proximal MCAO in the sheep results in space-occupying cerebral edema, raised ICP and mortality similar to human malignant MCA stroke. This animal model may prove useful for pre-clinical testing of anti-edema therapies that have shown promise in rodent studies.
Conflict of interest statement
Figures
Similar articles
-
A surgical model of permanent and transient middle cerebral artery stroke in the sheep.PLoS One. 2012;7(7):e42157. doi: 10.1371/journal.pone.0042157. Epub 2012 Jul 27. PLoS One. 2012. PMID: 22848737 Free PMC article.
-
[Follow-up monitoring with magnetic resonance tomography after decompressive trephining in experimental "malignant" hemispheric infarct].Zentralbl Neurochir. 1998;59(3):157-65. Zentralbl Neurochir. 1998. PMID: 9816666 German.
-
Malignant MCA Infarction: Pathophysiology and Imaging for Early Diagnosis and Management Decisions.Cerebrovasc Dis. 2016;41(1-2):1-7. doi: 10.1159/000441627. Epub 2015 Nov 19. Cerebrovasc Dis. 2016. PMID: 26581023 Review.
-
Aggravation of infarct formation by brain swelling in a large territorial stroke: a target for neuroprotection?J Neurosurg. 2008 Aug;109(2):287-93. doi: 10.3171/JNS/2008/109/8/0287. J Neurosurg. 2008. PMID: 18671642
-
Neuroprotection in malignant MCA infarction.Cerebrovasc Dis. 2006;21 Suppl 2:99-105. doi: 10.1159/000091709. Epub 2006 May 2. Cerebrovasc Dis. 2006. PMID: 16651820 Review.
Cited by
-
Determining the Temporal Profile of Intracranial Pressure Changes Following Transient Stroke in an Ovine Model.Front Neurosci. 2019 Jul 9;13:587. doi: 10.3389/fnins.2019.00587. eCollection 2019. Front Neurosci. 2019. PMID: 31338013 Free PMC article.
-
Nonhuman primate models of focal cerebral ischemia.Neural Regen Res. 2017 Feb;12(2):321-328. doi: 10.4103/1673-5374.200815. Neural Regen Res. 2017. PMID: 28400817 Free PMC article. Review.
-
Cerebrospinal fluid dynamics and intracranial pressure elevation in neurological diseases.Fluids Barriers CNS. 2019 Apr 10;16(1):9. doi: 10.1186/s12987-019-0129-6. Fluids Barriers CNS. 2019. PMID: 30967147 Free PMC article. Review.
-
Additive Effect of Resveratrol on Astrocyte Swelling Post-exposure to Ammonia, Ischemia and Trauma In Vitro.Neurochem Res. 2020 May;45(5):1156-1167. doi: 10.1007/s11064-020-02997-1. Epub 2020 Mar 12. Neurochem Res. 2020. PMID: 32166573
-
Characterization of tissue and functional deficits in a clinically translational pig model of acute ischemic stroke.Brain Res. 2020 Jun 1;1736:146778. doi: 10.1016/j.brainres.2020.146778. Epub 2020 Mar 16. Brain Res. 2020. PMID: 32194080 Free PMC article.
References
-
- Berrouschot J, Sterker M, Bettin S, Koster J, Schneider D. Mortality of space-occupying ('malignant') middle cerebral artery infarction under conservative intensive care. Intensive care medicine. 1998;24(6):620–3. Epub 1998/07/29. . - PubMed
-
- Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. 'Malignant' middle cerebral artery territory infarction: clinical course and prognostic signs. Archives of neurology. 1996;53(4):309–15. Epub 1996/04/01. . - PubMed
-
- Thrift AG, Dewey HM, Macdonell RA, McNeil JJ, Donnan GA. Stroke incidence on the east coast of Australia: the North East Melbourne Stroke Incidence Study (NEMESIS). Stroke; a journal of cerebral circulation. 2000;31(9):2087–92. Epub 2000/09/08. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

