Innate lymphoid cells in the initiation, regulation and resolution of inflammation
- PMID: 26121198
- PMCID: PMC4869856
- DOI: 10.1038/nm.3892
Innate lymphoid cells in the initiation, regulation and resolution of inflammation
Abstract
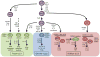
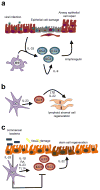
A previously unappreciated cell type of the innate immune system, termed innate lymphoid cells (ILCs), has been characterized in mice and humans and found to influence the induction, regulation and resolution of inflammation. ILCs have an important role in these processes in mouse models of infection, inflammation and tissue repair. Further, disease-association studies in defined patient populations have identified significant alterations in ILC responses, suggesting a potential role for these cell populations in human health and disease. In this review we discuss the emerging family of ILCs, the role of ILCs in inflammation, and how current or novel therapeutic strategies could be used to selectively modulate ILC responses and limit chronic inflammatory diseases.
Figures
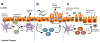


References
-
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. - PubMed
-
- Spits H, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. - PubMed
-
- Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells--how did we miss them? Nat Rev Immunol. 2013;13:75–87. - PubMed
-
- Sonnenberg GF, Mjosberg J, Spits H, Artis D. SnapShot: innate lymphoid cells. Immunity. 2013;39:622–622. e621. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 AI074878/AI/NIAID NIH HHS/United States
- R56 AI114724/AI/NIAID NIH HHS/United States
- R01 AI095466/AI/NIAID NIH HHS/United States
- AI061570/AI/NIAID NIH HHS/United States
- P01 AI106697/AI/NIAID NIH HHS/United States
- AI102942/AI/NIAID NIH HHS/United States
- R01 AI123368/AI/NIAID NIH HHS/United States
- DP5OD012116/OD/NIH HHS/United States
- DP5 OD012116/OD/NIH HHS/United States
- AI106697/AI/NIAID NIH HHS/United States
- AI074878/AI/NIAID NIH HHS/United States
- R01 AI102942/AI/NIAID NIH HHS/United States
- AI095608/AI/NIAID NIH HHS/United States
- UL1-RR024134/RR/NCRR NIH HHS/United States
- U01 AI095608/AI/NIAID NIH HHS/United States
- R01 AI061570/AI/NIAID NIH HHS/United States
- R01 AI097333/AI/NIAID NIH HHS/United States
- AI095466/AI/NIAID NIH HHS/United States
- UL1 RR024134/RR/NCRR NIH HHS/United States
- AI097333/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

