A FOXM1 Dependent Mesenchymal-Epithelial Transition in Retinal Pigment Epithelium Cells
- PMID: 26121260
- PMCID: PMC4488273
- DOI: 10.1371/journal.pone.0130379
A FOXM1 Dependent Mesenchymal-Epithelial Transition in Retinal Pigment Epithelium Cells
Abstract
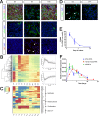
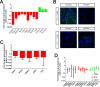
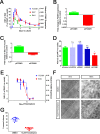
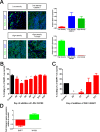
The integrity of the epithelium is maintained by a complex but regulated interplay of processes that allow conversion of a proliferative state into a stably differentiated state. In this study, using human embryonic stem cell (hESC) derived Retinal Pigment Epithelium (RPE) cells as a model; we have investigated the molecular mechanisms that affect attainment of the epithelial phenotype. We demonstrate that RPE undergo a Mesenchymal-Epithelial Transition in culture before acquiring an epithelial phenotype in a FOXM1 dependent manner. We show that FOXM1 directly regulates proliferation of RPE through transcriptional control of cell cycle associated genes. Additionally, FOXM1 modulates expression of the signaling ligands BMP7 and Wnt5B which act reciprocally to enable epithelialization. This data uncovers a novel effect of FOXM1 dependent activities in contributing towards epithelial fate acquisition and furthers our understanding of the molecular regulators of a cell type that is currently being evaluated as a cell therapy.
Conflict of interest statement
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

