Flow-dependent expression of ectonucleotide tri(di)phosphohydrolase-1 and suppression of atherosclerosis
- PMID: 26121751
- PMCID: PMC4563746
- DOI: 10.1172/JCI79514
Flow-dependent expression of ectonucleotide tri(di)phosphohydrolase-1 and suppression of atherosclerosis
Abstract
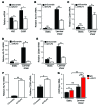
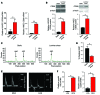
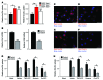
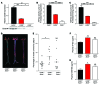
The ability of cells to detect and respond to nucleotide signals in the local microenvironment is essential for vascular homeostasis. The enzyme ectonucleotide tri(di)phosphohydrolase-1 (ENTPD1, also known as CD39) on the surface of leukocytes and endothelial cells metabolizes locally released, intravascular ATP and ADP, thereby eliminating these prothrombotic and proinflammatory stimuli. Here, we evaluated the contribution of CD39 to atherogenesis in the apolipoprotein E-deficient (ApoE-deficient) mouse model of atherosclerosis. Compared with control ApoE-deficient animals, plaque burden was markedly increased along with circulating markers of platelet activation in Cd39+/-Apoe-/- mice fed a high-fat diet. Plaque analysis revealed stark regionalization of endothelial CD39 expression and function in Apoe-/- mice, with CD39 prominently expressed in atheroprotective, stable flow regions and diminished in atheroprone areas subject to disturbed flow. In mice, disturbed flow as the result of partial carotid artery ligation rapidly suppressed endothelial CD39 expression. Moreover, unidirectional laminar shear stress induced atheroprotective CD39 expression in human endothelial cells. CD39 induction was dependent upon the vascular transcription factor Krüppel-like factor 2 (KLF2) binding near the transcriptional start site of CD39. Together, these data establish CD39 as a regionalized regulator of atherogenesis that is driven by shear stress.
Figures



References
-
- Huo Y, et al. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9(1):61–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL119798/HL/NHLBI NIH HHS/United States
- T32HL007853/HL/NHLBI NIH HHS/United States
- OD016502/OD/NIH HHS/United States
- K08 HL123621/HL/NHLBI NIH HHS/United States
- P01 HL095070/HL/NHLBI NIH HHS/United States
- T32 HL007853/HL/NHLBI NIH HHS/United States
- NS087147/NS/NINDS NIH HHS/United States
- L30 HL115712/HL/NHLBI NIH HHS/United States
- R01 NS087147/NS/NINDS NIH HHS/United States
- K26 OD016502/OD/NIH HHS/United States
- K08HL119623/HL/NHLBI NIH HHS/United States
- K23 HL119623/HL/NHLBI NIH HHS/United States
- HL127151/HL/NHLBI NIH HHS/United States
- K08HL123621/HL/NHLBI NIH HHS/United States
- R01 HL119798/HL/NHLBI NIH HHS/United States
- R01 HL127151/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

