Acceleration of protein folding by four orders of magnitude through a single amino acid substitution
- PMID: 26121966
- PMCID: PMC4485320
- DOI: 10.1038/srep11840
Acceleration of protein folding by four orders of magnitude through a single amino acid substitution
Abstract
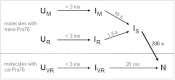
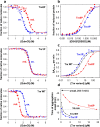
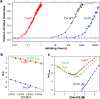
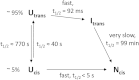
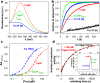
Cis prolyl peptide bonds are conserved structural elements in numerous protein families, although their formation is energetically unfavorable, intrinsically slow and often rate-limiting for folding. Here we investigate the reasons underlying the conservation of the cis proline that is diagnostic for the fold of thioredoxin-like thiol-disulfide oxidoreductases. We show that replacement of the conserved cis proline in thioredoxin by alanine can accelerate spontaneous folding to the native, thermodynamically most stable state by more than four orders of magnitude. However, the resulting trans alanine bond leads to small structural rearrangements around the active site that impair the function of thioredoxin as catalyst of electron transfer reactions by more than 100-fold. Our data provide evidence for the absence of a strong evolutionary pressure to achieve intrinsically fast folding rates, which is most likely a consequence of proline isomerases and molecular chaperones that guarantee high in vivo folding rates and yields.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

