Passive movement and active exercise for very young infants with congenital heart disease: a study protocol for a randomized controlled trial
- PMID: 26122088
- PMCID: PMC4485354
- DOI: 10.1186/s13063-015-0816-9
Passive movement and active exercise for very young infants with congenital heart disease: a study protocol for a randomized controlled trial
Abstract
Background: Delayed motor development is reported in patients with congenital heart disease (CHD). Exercise is widely used to facilitate motor development and improve motor ability. Exercise for adolescents and adults with CHD has been extensively studied. However, the evidence of exercise for infants with CHD is sparse. This study aims to identify the effect of passive movement and active exercise on motor development within very young CHD infants with cardiac catheterization.
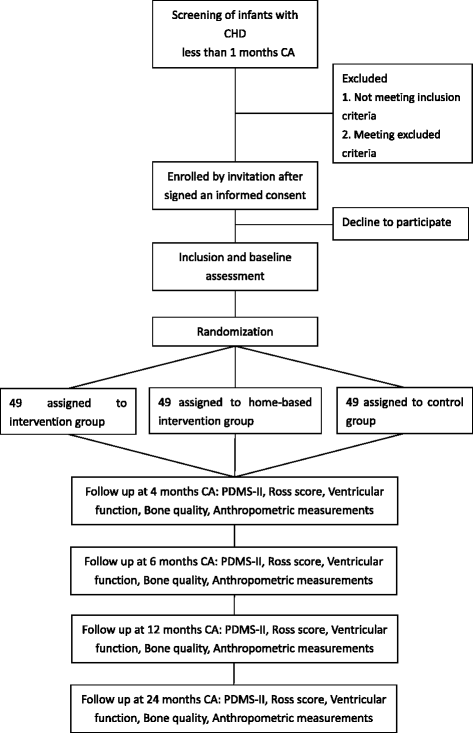
Methods/design: A prospective and randomized controlled trial will be conducted in very young CHD infants with cardiac catheterization. A total of 147 infants with CHD will be randomized by a 1:1:1 allocation ratio by computer to an exercise intervention group, a home-based intervention group and a control group. The exercise intervention group will receive passive movement and active exercise from experienced physiotherapists in pediatrics three times a week for 12 weeks. The home-based intervention group will receive passive movement and active exercise from their parents or caregivers at home three times a week for 12 weeks. The control group will receive follow up only. The follow-up duration is 20 months. The primary outcome measures are the motor quotient measured by the Peabody Developmental Motor Scales-II. The secondary outcome measures are the Ross score, ventricular function, bone quality, body length, weight, head circumference, upper arm circumference, and adverse events.
Discussion: This study has several important features, including the randomization process, the long follow-up duration, the control group, and the large sample size. The aim of this study is to determine whether 12-week passive movement and active exercise promotes motor development and produces other beneficial effects for very young CHD infants with cardiac catheterization. Therefore, this study will contribute new knowledge regarding the rehabilitation program in very young CHD infants with cardiac catheterization.
Trial registration: Current Controlled Trials ChiCTR-IOR-15005909 (January 31, 2015).
Figures
References
-
- Egbe A, Uppu S, Lee S, Stroustrup A, Ho D, Srivastava S. Temporal variation of birth prevalence of congenital heart disease in the United States. Congenit Heart Dis. 2014. doi: 10.1111/chd.12176. - PubMed
-
- Bhardwaj R, Rai SK, Yadav AK, Lakhotia S, Agrawal D, Kumar A, et al. Epidemiology of congenital heart disease in India. Congenit Heart Dis. 2014. doi: 10.1111/chd.12220. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical