Osteogenic changes in kidneys of hyperoxaluric rats
- PMID: 26122267
- PMCID: PMC4523408
- DOI: 10.1016/j.bbadis.2015.06.020
Osteogenic changes in kidneys of hyperoxaluric rats
Abstract
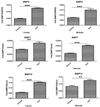
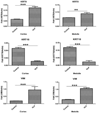
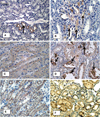
Many calcium oxalate (CaOx) kidney stones develop attached to renal papillary sub-epithelial deposits of calcium phosphate (CaP), called Randall's plaque (RP). Pathogenesis of the plaques is not fully understood. We hypothesize that abnormal urinary environment in stone forming kidneys leads to epithelial cells losing their identity and becoming osteogenic. To test our hypothesis male rats were made hyperoxaluric by administration of hydroxy-l-proline (HLP). After 28days, rat kidneys were extracted. We performed genome wide analyses of differentially expressed genes and determined changes consistent with dedifferentiation of epithelial cells into osteogenic phenotype. Selected molecules were further analyzed using quantitative-PCR and immunohistochemistry. Genes for runt related transcription factors (RUNX1 and 2), zinc finger protein Osterix, bone morphogenetic proteins (BMP2 and 7), bone morphogenetic protein receptor (BMPR2), collagen, osteocalcin, osteonectin, osteopontin (OPN), matrix-gla-protein (MGP), osteoprotegrin (OPG), cadherins, fibronectin (FN) and vimentin (VIM) were upregulated while those for alkaline phosphatase (ALP) and cytokeratins 10 and 18 were downregulated. In conclusion, epithelial cells of hyperoxaluric kidneys acquire a number of osteoblastic features but without CaP deposition, perhaps a result of downregulation of ALP and upregulation of OPN and MGP. Plaque formation may additionally require localized increases in calcium and phosphate and decrease in mineralization inhibitory potential.
Keywords: Bone morphogenetic protein; Hyperoxaluria; Matrix-gla-protein; Randall's plaque; Runt related transcription factor; Vimentin.
Published by Elsevier B.V.
Conflict of interest statement
The authors have declared that there is no conflict of interest.
Figures


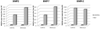






References
-
- Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int. 2003;63:1817–1823. - PubMed
-
- Kartha G, Calle JC, Marchini GS, Monga M. Impact of stone disease: chronic kidney disease and quality of life. Urol Clin North Am. 2013;40:135–147. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

