Minnelide, a novel drug for pancreatic and liver cancer
- PMID: 26122306
- PMCID: PMC4515388
- DOI: 10.1016/j.pan.2015.05.472
Minnelide, a novel drug for pancreatic and liver cancer
Abstract
Background: Pancreatic cancer is the 10th leading cause of all new cancer cases for men and the fourth leading cause of death across genders, having very poor prognosis and survival rates. The current standard of care Gemcitabine fails to add any survival benefit for this disease (www.cancer.gov). Though the incidence of pancreatic cancer is found to be higher in developed countries, the aggressive biology of the cancer, its high rate of recurrence and chemo-resistance make it a formidable disease in all parts of the globe. Hepatocellular carcinoma (HCC) or liver cancer, on the other hand affects almost 750,000 people world wide with 84% of the cases coming from underdeveloped or developing countries
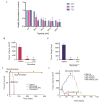
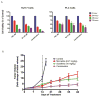
Results: Our studies show that Minnelide, a water soluble pro-drug of triptolide (active compound from a chinese herb) is very effective against a number of malignant diseases.
Conclusion: The current study discusses the efficacy of this compound in pancreatic and liver cancer.
Keywords: Animal models; Hepatocellular carcinoma; Minnelide; Pancreatic cancer; Translational study; Triptolide.
Copyright © 2015 IAP and EPC. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
University of Minnesota has filed a patent for Minnelide, which has been licensed to Minneamrita Therapeutics, LLC. AKS has financial interests in this company. SB is a consultant with Minneamrita Therapeutics.
Figures
Similar articles
-
Minnelide Overcomes Oxaliplatin Resistance by Downregulating the DNA Repair Pathway in Pancreatic Cancer.J Gastrointest Surg. 2016 Jan;20(1):13-23; discussion 23-4. doi: 10.1007/s11605-015-3000-3. Epub 2015 Oct 26. J Gastrointest Surg. 2016. PMID: 26503259 Free PMC article.
-
Pancreas cancer meets the thunder god.Sci Transl Med. 2012 Oct 17;4(156):156ps21. doi: 10.1126/scitranslmed.3004956. Sci Transl Med. 2012. PMID: 23076355
-
Primary and liver metastasis-derived cell lines from KrasG12D; Trp53R172H; Pdx-1 Cre animals undergo apoptosis in response to triptolide.Pancreas. 2015 May;44(4):583-9. doi: 10.1097/MPA.0000000000000317. Pancreas. 2015. PMID: 25875797 Free PMC article.
-
Triptolide and Its Derivatives as Cancer Therapies.Trends Pharmacol Sci. 2019 May;40(5):327-341. doi: 10.1016/j.tips.2019.03.002. Epub 2019 Apr 8. Trends Pharmacol Sci. 2019. PMID: 30975442 Review.
-
Triptolide: A new star for treating human malignancies.J Cancer Res Ther. 2018 Jun;14(Supplement):S271-S275. doi: 10.4103/0973-1482.235340. J Cancer Res Ther. 2018. PMID: 29970675 Review.
Cited by
-
The Integrated Stress Response in Pancreatic Development, Tissue Homeostasis, and Cancer.Gastroenterology. 2024 Dec;167(7):1292-1306. doi: 10.1053/j.gastro.2024.05.009. Epub 2024 May 18. Gastroenterology. 2024. PMID: 38768690 Review.
-
Therapeutic potential of Triptolide in inhibiting breast cancer-induced bone destruction - PTHrP as a therapeutic target.Front Pharmacol. 2025 May 15;16:1512631. doi: 10.3389/fphar.2025.1512631. eCollection 2025. Front Pharmacol. 2025. PMID: 40444046 Free PMC article.
-
NQO1-Selective Activated Prodrug of Triptolide: Synthesis and Antihepatocellular Carcinoma Activity Evaluation.ACS Med Chem Lett. 2018 Nov 27;9(12):1253-1257. doi: 10.1021/acsmedchemlett.8b00404. eCollection 2018 Dec 13. ACS Med Chem Lett. 2018. PMID: 30613335 Free PMC article.
-
Reciprocal Regulation of Hippo and WBP2 Signalling-Implications in Cancer Therapy.Cells. 2021 Nov 11;10(11):3130. doi: 10.3390/cells10113130. Cells. 2021. PMID: 34831354 Free PMC article. Review.
-
Transcription and Translation Inhibitors in Cancer Treatment.Front Chem. 2020 Apr 21;8:276. doi: 10.3389/fchem.2020.00276. eCollection 2020. Front Chem. 2020. PMID: 32373584 Free PMC article. Review.
References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA: a cancer journal for clinicians. 2010;60(5):277–300. - PubMed
-
- Lowenfels AB, Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best practice & research Clinical gastroenterology. 2006;20(2):197–209. - PubMed
-
- O’Sullivan A, Kocher HM. Pancreatic cancer. Clinical evidence. 2007 - PubMed
-
- Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363(9414):1049–1057. - PubMed
-
- McCracken M, Olsen M, Chen MS, Jr, Jemal A, Thun M, Cokkinides V, Deapen D, Ward E. Cancer incidence, mortality, and associated risk factors among Asian Americans of Chinese, Filipino, Vietnamese, Korean, and Japanese ethnicities. CA Cancer J Clin. 2007;57(4):190–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

