Chronic Rhinosinusitis and the Coagulation System
- PMID: 26122502
- PMCID: PMC4509654
- DOI: 10.4168/aair.2015.7.5.421
Chronic Rhinosinusitis and the Coagulation System
Abstract
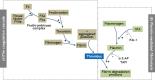
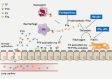
Chronic rhinosinusitis (CRS) is one of the most common chronic diseases in adults and severely affects quality of life in patients. Although various etiologic and pathogenic mechanisms of CRS have been proposed, the causes of CRS remain uncertain. Abnormalities in the coagulation cascade may play an etiologic role in many diseases, such as asthma and other inflammatory conditions. While studies on the relationship between asthma and dysregulated coagulation have been reported, the role of the coagulation system in the pathogenesis of CRS has only been considered following recent reports. Excessive fibrin deposition is seen in nasal polyp (NP) tissue from patients with chronic rhinosinusitis with nasal polyp (CRSwNP) and is associated with activation of thrombin, reduction of tissue plasminogen activator (t-PA) and upregulation of coagulation factor XIII-A (FXIII-A), all events that can contribute to fibrin deposition and crosslinking. These findings were reproduced in a murine model of NP that was recently established. Elucidation of the mechanisms of fibrin deposition may enhance our understanding of tissue remodeling in the pathophysiology of NP and provide new targets for the treatment of CRSwNP.
Keywords: Rhinosinusitis; coagulation; factor XIIIa; fibrinolysis; nasal polyps; tissue plasminogen activator.
Conflict of interest statement
There are no financial or other issues that might lead to conflict of interest.
Figures


Similar articles
-
Studies on activation and regulation of the coagulation cascade in chronic rhinosinusitis with nasal polyps.J Allergy Clin Immunol. 2022 Aug;150(2):467-476.e1. doi: 10.1016/j.jaci.2022.02.018. Epub 2022 Mar 7. J Allergy Clin Immunol. 2022. PMID: 35271862 Free PMC article.
-
Increased expression of factor XIII-A in patients with chronic rhinosinusitis with nasal polyps.J Allergy Clin Immunol. 2013 Sep;132(3):584-592.e4. doi: 10.1016/j.jaci.2013.02.003. Epub 2013 Mar 28. J Allergy Clin Immunol. 2013. PMID: 23541322 Free PMC article.
-
Excessive fibrin deposition in nasal polyps caused by fibrinolytic impairment through reduction of tissue plasminogen activator expression.Am J Respir Crit Care Med. 2013 Jan 1;187(1):49-57. doi: 10.1164/rccm.201207-1292OC. Epub 2012 Nov 15. Am J Respir Crit Care Med. 2013. PMID: 23155140 Free PMC article.
-
Classification of chronic rhinosinusitis according to a nasal polyp and tissue eosinophilia: limitation of current classification system for Asian population.Asia Pac Allergy. 2017 Jul;7(3):121-130. doi: 10.5415/apallergy.2017.7.3.121. Epub 2017 Jul 26. Asia Pac Allergy. 2017. PMID: 28765816 Free PMC article. Review.
-
Pathophysiology of chronic rhinosinusitis with nasal polyp.Am J Rhinol Allergy. 2011 Sep-Oct;25(5):285-90. doi: 10.2500/ajra.2011.25.3680. Am J Rhinol Allergy. 2011. PMID: 22186239 Review.
Cited by
-
Sinonasal Tissue Remodelling during Chronic Rhinosinusitis.Int J Otolaryngol. 2021 Sep 16;2021:7428955. doi: 10.1155/2021/7428955. eCollection 2021. Int J Otolaryngol. 2021. PMID: 34567126 Free PMC article. Review.
-
Studies on activation and regulation of the coagulation cascade in chronic rhinosinusitis with nasal polyps.J Allergy Clin Immunol. 2022 Aug;150(2):467-476.e1. doi: 10.1016/j.jaci.2022.02.018. Epub 2022 Mar 7. J Allergy Clin Immunol. 2022. PMID: 35271862 Free PMC article.
-
The Role of Exosomes in the Pathophysiology of Chronic Rhinosinusitis.Front Cell Infect Microbiol. 2022 Jan 28;11:812920. doi: 10.3389/fcimb.2021.812920. eCollection 2021. Front Cell Infect Microbiol. 2022. PMID: 35155273 Free PMC article. Review.
-
Lessons From Localized Chronic Rhinosinusitis With Nasal Polyps.Allergy Asthma Immunol Res. 2021 Nov;13(6):827-829. doi: 10.4168/aair.2021.13.6.827. Allergy Asthma Immunol Res. 2021. PMID: 34734501 Free PMC article. No abstract available.
-
Activation of the Coagulation Cascade as a Universal Danger Sign.Curr Issues Mol Biol. 2025 Feb 9;47(2):108. doi: 10.3390/cimb47020108. Curr Issues Mol Biol. 2025. PMID: 39996829 Free PMC article. Review.
References
-
- Van Bruaene N, Derycke L, Perez-Novo CA, Gevaert P, Holtappels G, De Ruyck N, et al. TGF-beta signaling and collagen deposition in chronic rhinosinusitis. J Allergy Clin Immunol. 2009;124:253–259. 259.e1–259.e2. - PubMed
-
- Bachert C, Gevaert P, van Cauwenberge P. Staphylococcus aureus superantigens and airway disease. Curr Allergy Asthma Rep. 2002;2:252–258. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

