Proteostasis control by the unfolded protein response
- PMID: 26123108
- PMCID: PMC5546321
- DOI: 10.1038/ncb3184
Proteostasis control by the unfolded protein response
Erratum in
-
Erratum: Proteostasis control by the unfolded protein response.Nat Cell Biol. 2015 Aug;17(8):1088. doi: 10.1038/ncb3221. Nat Cell Biol. 2015. PMID: 26239530 Free PMC article.
Abstract
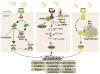
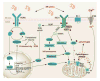
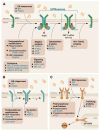
Stress induced by accumulation of misfolded proteins in the endoplasmic reticulum is observed in many physiological and pathological conditions. To cope with endoplasmic reticulum stress, cells activate the unfolded protein response, a dynamic signalling network that orchestrates the recovery of homeostasis or triggers apoptosis, depending on the level of damage. Here we provide an overview of recent insights into the mechanisms that cells employ to maintain proteostasis and how the unfolded protein response determines cell fate under endoplasmic reticulum stress.
Figures

References
-
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. - PubMed
-
- Schubert U, et al. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–774. - PubMed
-
- Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332:462–464. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

