Regulation of the transient receptor potential channel TRPM3 by phosphoinositides
- PMID: 26123194
- PMCID: PMC4485019
- DOI: 10.1085/jgp.201411339
Regulation of the transient receptor potential channel TRPM3 by phosphoinositides
Abstract
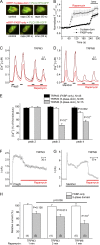
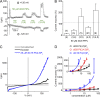
The transient receptor potential (TRP) channel TRPM3 is a calcium-permeable cation channel activated by heat and by the neurosteroid pregnenolone sulfate (PregS). TRPM3 is highly expressed in sensory neurons, where it plays a key role in heat sensing and inflammatory hyperalgesia, and in pancreatic β cells, where its activation enhances glucose-induced insulin release. However, despite its functional importance, little is known about the cellular mechanisms that regulate TRPM3 activity. Here, we provide evidence for a dynamic regulation of TRPM3 by membrane phosphatidylinositol phosphates (PIPs). Phosphatidylinositol 4,5-bisphosphate (PI[4,5]P2) and ATP applied to the intracellular side of excised membrane patches promote recovery of TRPM3 from desensitization. The stimulatory effect of cytosolic ATP on TRPM3 reflects activation of phosphatidylinositol kinases (PI-Ks), leading to resynthesis of PIPs in the plasma membrane. Various PIPs directly enhance TRPM3 activity in cell-free inside-out patches, with a potency order PI(3,4,5)P3 > PI(3,5)P2 > PI(4,5)P2 ≈ PI(3,4)P2 >> PI(4)P. Conversely, TRPM3 activity is rapidly and reversibly inhibited by activation of phosphatases that remove the 5-phosphate from PIPs. Finally, we show that recombinant TRPM3, as well as the endogenous TRPM3 in insuloma cells, is rapidly and reversibly inhibited by activation of phospholipase C-coupled muscarinic acetylcholine receptors. Our results reveal basic cellular mechanisms whereby membrane receptors can regulate TRPM3 activity.
© 2015 Tóth et al.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

