Transient receptor potential melastatin 3 is a phosphoinositide-dependent ion channel
- PMID: 26123195
- PMCID: PMC4485020
- DOI: 10.1085/jgp.201411336
Transient receptor potential melastatin 3 is a phosphoinositide-dependent ion channel
Abstract
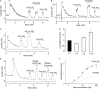
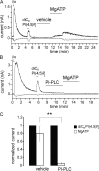
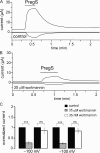
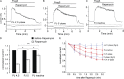
Phosphoinositides are emerging as general regulators of the functionally diverse transient receptor potential (TRP) ion channel family. Phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) has been reported to positively regulate many TRP channels, but in several cases phosphoinositide regulation is controversial. TRP melastatin 3 (TRPM3) is a heat-activated ion channel that is also stimulated by chemical agonists, such as pregnenolone sulfate. Here, we used a wide array of approaches to determine the effects of phosphoinositides on TRPM3. We found that channel activity in excised inside-out patches decreased over time (rundown), an attribute of PI(4,5)P2-dependent ion channels. Channel activity could be restored by application of either synthetic dioctanoyl (diC8) or natural arachidonyl stearyl (AASt) PI(4,5)P2. The PI(4,5)P2 precursor phosphatidylinositol 4-phosphate (PI(4)P) was less effective at restoring channel activity. TRPM3 currents were also restored by MgATP, an effect which was inhibited by two different phosphatidylinositol 4-kinase inhibitors, or by pretreatment with a phosphatidylinositol-specific phospholipase C (PI-PLC) enzyme, indicating that MgATP acted by generating phosphoinositides. In intact cells, reduction of PI(4,5)P2 levels by chemically inducible phosphoinositide phosphatases or a voltage-sensitive 5'-phosphatase inhibited channel activity. Activation of PLC via muscarinic receptors also inhibited TRPM3 channel activity. Overall, our data indicate that TRPM3 is a phosphoinositide-dependent ion channel and that decreasing PI(4,5)P2 abundance limits its activity. As all other members of the TRPM family have also been shown to require PI(4,5)P2 for activity, our data establish PI(4,5)P2 as a general positive cofactor of this ion channel subfamily.
© 2015 Badheka et al.
Figures



Comment in
-
TRPM3 joins the ranks of PI(4,5)P2 sensitive ion channels.Channels (Austin). 2015;9(5):233-4. doi: 10.1080/19336950.2015.1089072. Channels (Austin). 2015. PMID: 26542627 Free PMC article.
References
-
- Bojjireddy N., Botyanszki J., Hammond G., Creech D., Peterson R., Kemp D.C., Snead M., Brown R., Morrison A., Wilson S., et al. . 2014. Pharmacological and genetic targeting of the PI4KA enzyme reveals its important role in maintaining plasma membrane phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate levels. J. Biol. Chem. 289:6120–6132. 10.1074/jbc.M113.531426 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

