Role of CD4+ Foxp3+ Regulatory T Cells in Protection Induced by a Live Attenuated, Replicating Type I Vaccine Strain of Toxoplasma gondii
- PMID: 26123802
- PMCID: PMC4534668
- DOI: 10.1128/IAI.00217-15
Role of CD4+ Foxp3+ Regulatory T Cells in Protection Induced by a Live Attenuated, Replicating Type I Vaccine Strain of Toxoplasma gondii
Abstract
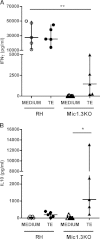
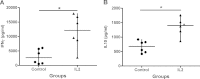
Vaccination with the live attenuated Toxoplasma gondii Mic1.3KO strain induced long-lasting immunity against challenge with Toxoplasma gondii type I and type II strains. The involvement of regulatory T cells (Tregs) in the protection mechanism was investigated. Intraperitoneal injection of Mic1.3KO induced a weak and transient influx of CD4(+) Foxp3(+) T regulatory cells followed by recruitment/expansion of CD4(+) Foxp3(-) CD25(+) effector cells and control of the parasite at the site of infection. The local and systemic cytokine responses associated with this recruitment of Tregs were of the TH1/Treg-like type. In contrast, injection of RH, the wild-type strain from which the vaccinal strain is derived, induced a low CD4(+) Foxp3(+) cell influx and uncontrolled multiplication of the parasites at this local site, followed by death of the mice. The associated local and systemic cytokine responses were of the TH1/TH17-like type. In addition, in vivo Treg induction in RH-infected mice with interleukin-2 (IL-2)/anti-IL-2 complexes induced control of the parasite and a TH1/Treg cytokine response similar to the response after Mic1.3KO vaccination. These results suggest that Tregs may contribute to the protective response after vaccination with Mic1.3KO.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures





Similar articles
-
Mic1-3 knockout of Toxoplasma gondii is a successful vaccine against chronic and congenital toxoplasmosis in mice.J Infect Dis. 2006 Oct 15;194(8):1176-83. doi: 10.1086/507706. Epub 2006 Sep 11. J Infect Dis. 2006. PMID: 16991094
-
Mic1-3KO tachyzoite a live attenuated vaccine candidate against toxoplasmosis derived from a type I strain shows features of type II strain.Exp Parasitol. 2009 Oct;123(2):111-7. doi: 10.1016/j.exppara.2009.06.003. Epub 2009 Jun 7. Exp Parasitol. 2009. PMID: 19508866
-
Evaluation of immunogenicity and protection of the Mic1-3 knockout Toxoplasma gondii live attenuated strain in the feline host.Vaccine. 2020 Feb 5;38(6):1457-1466. doi: 10.1016/j.vaccine.2019.11.076. Epub 2019 Dec 18. Vaccine. 2020. PMID: 31864855
-
Vaccination as a control strategy against the coccidial parasites Eimeria, Toxoplasma and Neospora.Parasitology. 2006;133 Suppl:S145-68. doi: 10.1017/S0031182006001855. Parasitology. 2006. PMID: 17274844 Review.
-
Protective immunity against lethal anaphylactic reaction in Toxoplasma gondii-infected mice by DNA vaccination with T. gondii-derived heat shock protein 70 gene.Parasitol Int. 2010 Jun;59(2):105-11. doi: 10.1016/j.parint.2010.03.006. Epub 2010 Mar 24. Parasitol Int. 2010. PMID: 20346412 Review.
Cited by
-
Acetaminophen, a new tool for the refinement of the experimental infection of toxoplasmosis in mice.Sci Rep. 2025 Jul 1;15(1):22157. doi: 10.1038/s41598-025-06849-2. Sci Rep. 2025. PMID: 40594552 Free PMC article.
-
Immune response against toxoplasmosis-some recent updates RH: Toxoplasma gondii immune response.Int J Immunopathol Pharmacol. 2022 Jan-Dec;36:3946320221078436. doi: 10.1177/03946320221078436. Int J Immunopathol Pharmacol. 2022. PMID: 35227108 Free PMC article. Review.
-
Is Toxoplasma gondii type related to clinical outcome in human congenital infection? Systematic and critical review.Eur J Clin Microbiol Infect Dis. 2016 Jul;35(7):1079-88. doi: 10.1007/s10096-016-2656-2. Epub 2016 May 4. Eur J Clin Microbiol Infect Dis. 2016. PMID: 27146878
-
Immunization with Toxoplasma gondii GRA17 Deletion Mutant Induces Partial Protection and Survival in Challenged Mice.Front Immunol. 2017 Jun 29;8:730. doi: 10.3389/fimmu.2017.00730. eCollection 2017. Front Immunol. 2017. PMID: 28706518 Free PMC article.
-
Treatment of mice with S4B6 IL-2 complex prevents lethal toxoplasmosis via IL-12- and IL-18-dependent interferon-gamma production by non-CD4 immune cells.Sci Rep. 2020 Aug 4;10(1):13115. doi: 10.1038/s41598-020-70102-1. Sci Rep. 2020. PMID: 32753607 Free PMC article.
References
-
- Gigley JP, Fox BA, Bzik DJ. 2009. Long-term immunity to lethal acute or chronic type II Toxoplasma gondii infection is effectively induced in genetically susceptible C57BL/6 mice by immunization with an attenuated type I vaccine strain. Infect Immun 77:5380–5388. doi:10.1128/IAI.00649-09. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

