Drug-eluting microarrays to identify effective chemotherapeutic combinations targeting patient-derived cancer stem cells
- PMID: 26124098
- PMCID: PMC4507198
- DOI: 10.1073/pnas.1505374112
Drug-eluting microarrays to identify effective chemotherapeutic combinations targeting patient-derived cancer stem cells
Abstract
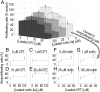
A new paradigm in oncology establishes a spectrum of tumorigenic potential across the heterogeneous phenotypes within a tumor. The cancer stem cell hypothesis postulates that a minute fraction of cells within a tumor, termed cancer stem cells (CSCs), have a tumor-initiating capacity that propels tumor growth. An application of this discovery is to target this critical cell population using chemotherapy; however, the process of isolating these cells is arduous, and the rarity of CSCs makes it difficult to test potential drug candidates in a robust fashion, particularly for individual patients. To address the challenge of screening drug libraries on patient-derived populations of rare cells, such as CSCs, we have developed a drug-eluting microarray, a miniaturized platform onto which a minimal quantity of cells can adhere and be exposed to unique treatment conditions. Hundreds of drug-loaded polymer islands acting as drug depots colocalized with adherent cells are surrounded by a nonfouling background, creating isolated culture environments on a solid substrate. Significant results can be obtained by testing <6% of the cells required for a typical 96-well plate. Reliability was demonstrated by an average coefficient of variation of 14% between all of the microarrays and 13% between identical conditions within a single microarray. Using the drug-eluting array, colorectal CSCs isolated from two patients exhibited unique responses to drug combinations when cultured on the drug-eluting microarray, highlighting the potential as a prognostic tool to identify personalized chemotherapeutic regimens targeting CSCs.
Keywords: cancer stem cell; chemopredictive; combination therapy; microarray; personalized medicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. - PubMed
-
- Huang EH, Heidt DG, Li CW, Simeone DM. Cancer stem cells: A new paradigm for understanding tumor progression and therapeutic resistance. Surgery. 2007;141(4):415–419. - PubMed
-
- Flaim CJ, Chien S, Bhatia SN. An extracellular matrix microarray for probing cellular differentiation. Nat Methods. 2005;2(2):119–125. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

