Massive accumulation of luminal protease-deficient axonal lysosomes at Alzheimer's disease amyloid plaques
- PMID: 26124111
- PMCID: PMC4507205
- DOI: 10.1073/pnas.1510329112
Massive accumulation of luminal protease-deficient axonal lysosomes at Alzheimer's disease amyloid plaques
Abstract
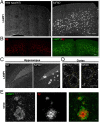
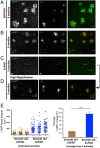
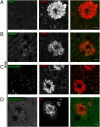
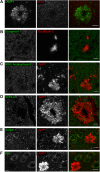
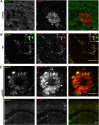
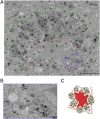
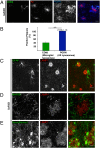
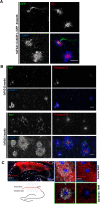
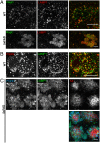
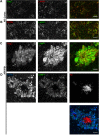
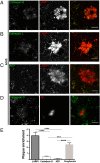
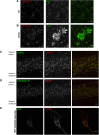
Through a comprehensive analysis of organellar markers in mouse models of Alzheimer's disease, we document a massive accumulation of lysosome-like organelles at amyloid plaques and establish that the majority of these organelles reside within swollen axons that contact the amyloid deposits. This close spatial relationship between axonal lysosome accumulation and extracellular amyloid aggregates was observed from the earliest stages of β-amyloid deposition. Notably, we discovered that lysosomes that accumulate in such axons are lacking in multiple soluble luminal proteases and thus are predicted to be unable to efficiently degrade proteinaceous cargos. Of relevance to Alzheimer's disease, β-secretase (BACE1), the protein that initiates amyloidogenic processing of the amyloid precursor protein and which is a substrate for these proteases, builds up at these sites. Furthermore, through a comparison between the axonal lysosome accumulations at amyloid plaques and neuronal lysosomes of the wild-type brain, we identified a similar, naturally occurring population of lysosome-like organelles in neuronal processes that is also defined by its low luminal protease content. In conjunction with emerging evidence that the lysosomal maturation of endosomes and autophagosomes is coupled to their retrograde transport, our results suggest that extracellular β-amyloid deposits cause a local impairment in the retrograde axonal transport of lysosome precursors, leading to their accumulation and a blockade in their further maturation. This study both advances understanding of Alzheimer's disease brain pathology and provides new insights into the subcellular organization of neuronal lysosomes that may have broader relevance to other neurodegenerative diseases with a lysosomal component to their pathology.
Keywords: Alzheimer's; axonal transport; cathepsin; lysosome; progranulin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Selkoe DJ. SnapShot: Pathobiology of Alzheimer's disease. Cell. 2013;154(2):468–468.e1. - PubMed
-
- Karran E, Mercken M, De Strooper B. The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat Rev Drug Discov. 2011;10(9):698–712. - PubMed
-
- Tanzi RE, Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 2005;120(4):545–555. - PubMed
-
- Jonsson T, et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature. 2012;488(7409):96–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

